Abstract
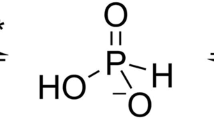
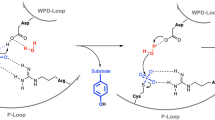
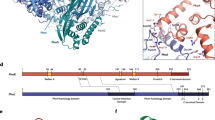
Arsenate reductase (ArsC) from Staphylococcus aureus plasmid pI258 plays a role in bacterial heavy metal resistance and catalyzes the reduction of arsenate to arsenite. The structures of the oxidized and reduced forms of ArsC were solved. ArsC has the PTPase I fold typical for low molecular weight tyrosine phosphatases (LMW PTPases). Remarkably, kinetic experiments show that pI258 ArsC also catalyzes the tyrosine phosphatase reaction in addition to arsenate reduction. These results provide evidence that ArsC from pI258 evolved from LMW PTPase by the grafting of a redox function onto a pre-existing catalytic site and that its evolutionary origin is different from those of arsenate reductases from Escherichia coli plasmid R773 and from Saccharomyces cerevisiae. The mechanism proposed here for the catalysis of arsenate reduction by pI258 ArsC involves a nucleophilic attack by Cys 10 on arsenate, the formation of a covalent intermediate and the transport of oxidative equivalents by a disulfide cascade. The reaction is associated with major structural changes in the ArsC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Babbitt, P.C. & Gerlt, J.A. Adv. Protein Chem. 55, 1–28 (2000).
Hasson, M.S. et al. Proc. Natl. Acad. Sci. USA 95, 10396–10401 (1998).
Altamirano, M.M., Blackburn, J.M., Aguayo, C. & Fersht, A.R. Nature 403, 617–622 (2000).
Silver, S. et al. Mol. Microbiol. 8, 637–642 (1993).
Ji, G. & Silver, S. J. Bacteriol. 174, 3684–3694 (1992).
Rosenstein, R., Peschel, A., Wieland, B. & Gotz, F. J. Bacteriol. 174, 3676–3683 (1992).
Messens, J., Hayburn, G., Desmyter, A., Laus, G. & Wyns, L. Biochemistry 38, 16857–16865 (1999).
Ji, G. et al. Biochemistry 33, 7294–7299 (1994).
Stevens, S.Y. et al. Biochemistry 38, 10178–10186 (1999).
Mukhopadhyay, R., Shi, J. & Rosen, B.P. J. Biol. Chem. 275, 21149–21157 (2000).
Shi, J., Vlamis-Gardikas, A., Aslund, F., Holmgren, A. & Rosen, B.P. J. Biol. Chem. 274, 36039–36042 (1999).
Shi, L., Potts, M. & Kennelly, P.J. FEMS Microbiol. Rev. 22, 229–253 (1998).
Denu, J.M. & Dixon, J.E. Curr. Opin. Chem. Biol. 2, 633–641 (1998).
Ramponi, G. & Stefani, M. Biochim. Biophys. Acta 1341, 137–156 (1997).
Zhang, M., Stauffacher, C.V., Lin, D. & Van Etten, R.L. J. Biol. Chem. 273, 21714–21720 (1998).
Messens, J. et al. J. Biol. Inorg. Chem. In the press. (2001).
Jeffery, C.J. Trends Biochem. Sci. 24, 8–11 (1999).
Hunter, T. Cell 80, 225–236 (1995).
Kennelly, P.J. & Potts, M. Front. Biosci. 4, 372–385 (1999).
Zhang, Y.L. & Zhang, Z.Y. Anal. Biochem. 261, 139–148 (1998).
Rusnak, F. & Reiter, T. Trends Biochem. Sci. 25, 527–529 (2000).
Ab, E. et al. Protein Sci. 6, 304–314 (1997).
Logan, T.M. et al. Biochemistry 33, 11087–11096 (1994).
Lennon, B.W., Williams, C.H. & Ludwig, M.L. Science 289, 1190–1194 (2000).
Katzen, F. & Beckwith, J. Cell 103, 769–779 (2000).
Budisa, N. et al. Eur. J. Biochem. 230, 788–796 (1995).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 50, 760–763 (1994).
Terwilliger, T.C. & Berendzen, J. Acta Crystallogr. D 55, 849–861 (1999).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Brunger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Esnouf, R.M. J. Mol. Graph. Model. 15, 132–133 (1997).
Acknowledgements
We wish to thank R. Loris and D. Maes for help with data collection, K. Van Belle for the purification of the SeMet ArsC and A. Desmyter for the construction of the C89L mutant of pI258 ArsC. We are also grateful to P. Tucker for help with the MAD datacollection and Y. Geunes for help with figures. We thank S. Silver of the University of Illinois College of Medicine, Chicago, for providing us with the arsC gene of plasmid pI258. We thank the ESA program Prodex for funding for the study of crystallization processes. This work was funded in part by the VIB, Vlaams Interuniversitair instituut voor Biotechnologie and the Fund for Scientific Research Flanders (F.W.O.) (Belgium) and the Research Council of the VUB. J.C.M. is a Postdoctoral Fellow of the F.W.O.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zegers, I., Martins, J., Willem, R. et al. Arsenate reductase from S. aureus plasmid pI258 is a phosphatase drafted for redox duty. Nat Struct Mol Biol 8, 843–847 (2001). https://doi.org/10.1038/nsb1001-843
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1001-843
This article is cited by
-
Self-assembling thermostable chimeras as new platform for arsenic biosensing
Scientific Reports (2021)
-
Intra- and inter-protein couplings of backbone motions underlie protein thiol-disulfide exchange cascade
Scientific Reports (2018)
-
Functional analysis of ars gene cluster of Pannonibacter indicus strain HT23T (DSM 23407T) and identification of a proline residue essential for arsenate reductase activity
Applied Microbiology and Biotechnology (2016)
-
Relative Expression of Low Molecular Weight Protein, Tyrosine Phosphatase (Wzb Gene) of Herbaspirillum sp. GW103 Toward Arsenic Stress and Molecular Modeling
Current Microbiology (2015)
-
Bacterial metabolism of environmental arsenic—mechanisms and biotechnological applications
Applied Microbiology and Biotechnology (2013)