Abstract
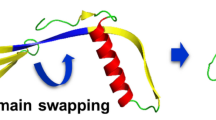
Amyloid precursor protein (APP) plays a central role in Alzheimer disease. A proteolytic-breakdown product of APP, called β-amyloid, is a major component of the diffuse and fibrillar deposits found in Alzheimer diseased brains. The normal physiological role of APP remains largely unknown despite much work. A knowledge of its function will not only provide insights into the genesis of the disease but may also prove vital in the development of an effective therapy. Here we describe the 1.8 Å resolution crystal structure of the N-terminal, heparin-binding domain of APP (residues 28–123), which is responsible, among other things, for stimulation of neurite outgrowth. The structure reveals a highly charged basic surface that may interact with glycosaminoglycans in the brain and an abutting hydrophobic surface that is proposed to play an important functional role such as dimerization or ligand binding. Structural similarities with cysteine-rich growth factors, taken together with its known growth-promoting properties, suggests the APP N-terminal domain could function as a growth factor in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Mattson, M.P. Physiol. Rev. 77, 1081–1132 (1997).
Kang, J. et al. Nature 325, 733–736 (1987).
Okamoto, T. et al. EMBO J. 15, 3769–3777 (1996).
Zambrano, N. et al. J. Biol. Chem. 272, 6399– 6405 (1997).
Small, D.H et al. J. Neurosci. 14, 2117– 2127 (1994).
Morimoto, T., Ohsawa, I., Takamura, C., Ishiguro, M. & Kohsaka, S. J. Neurosci. Res. 51, 185– 195 (1998).
Okamoto, T., Takeda, S., Murayama, Y., Ogata, E. & Nishimoto, I. J. Biol. Chem. 270, 4205– 4208 (1995).
Murayama, Y. et al. Gerontology 42, 2– 11 (1996).
Leveugle, B. et al. Neurochem. Int. 30, 543– 548 (1997).
Fenton, H. et al. Brain Res. 779, 262– 270 (1998).
Leveugle, B. et al. J. Neurochem. 70, 736– 744 (1998).
Greenberg, S.M., Qiu, W.Q., Selkoe, D.J., Ben-Itzhak, A. & Kosik, K.S. Neurosci. Lett. 198, 52– 56 (1995).
Bush, A.I., Pettingell, W.H., de Paradis, M., Tanzi, R.E. & Wasco, W. J. Biol. Chem. 269, 26618–26621 (1994).
Narindrasorasak, S. et al. J. Biol. Chem. 266, 12878– 12883 (1991).
Williamson, T.G., Nurcombe, V., Beyreuther, K., Masters, C.L. & Small, D.H. J. Neurochem. 65, 2201–2208 (1995).
Williamson, T.G. et al. J. Biol. Chem. 271, 31215– 31221 (1996).
Ohsawa, I., Takamura, C. & Kohsaka, S. Biochem. Biophys. Res. Commun. 236, 59–65 (1997).
Spencer, D.M., Wandless, T.J., Schreiber, S.L. & Crabtree, G.R. Science 262, 1019–1024 ( 1993).
Iwasaki, W. et al. EMBO J. 16, 6936–6946 (1997).
Zhou, H. et al. Structure 6, 109–116 (1998).
DiGabriele, A.D. et al. Nature 393, 812–817 (1998).
Holm, L. & Sander, C. J. Mol. Biol. 233, 123–138 (1993).
Fairbrother, W.J., Champe, M.A., Christinger, H.W., Keyt, B.A. & Starovasnik, M.A. Structure 6, 637–648 (1998).
Pietrzik, C.U. et al. Proc. Natl. Acad. Sci. USA 95, 1770 –1775 (1998).
Akar, C.A. & Wallace, W.C. Mol. Brain. Res. 56 , 125–132 (1998).
Henry, A., Masters, C.L., Beyreuther, K. & Cappai, R. Protein Expr. Purif. 10, 283–291 (1997).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 ( 1997).
Lüthy, R., Bowie, J.U. & Eisenberg, D. Nature 356, 83– 85 (1992).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Nicholls, A., Sharp, K.A. & Honig, B. Prot. Struct. Funct. Genet. 11, 281–293 (1991).
Barton, G.J. Protein Eng. 6, 37–40 ( 1993).
Hilbich, C., Mönning, U., Grund, C., Masters, C.L. & Beyreuther, K. J. Biol. Chem. 268 , 26571–26577 (1993).
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council of Australia to C.L.M. and M.W.P. K.B. is supported by the Deutsche Forschungsgemeinschaft and the Bundesministerium für Forschung und Technologie. J.R. is an Australian Research Council Postdoctoral Fellow, S.C.F. holds an International Centre for Diffraction Data Crystallography Scholarship and M.W.P. is an Australian Research Council Senior Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossjohn, J., Cappai, R., Feil, S. et al. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Mol Biol 6, 327–331 (1999). https://doi.org/10.1038/7562
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7562
This article is cited by
-
A tailored tetravalent peptide displays dual functions to inhibit amyloid β production and aggregation
Communications Biology (2023)
-
Structural biology of cell surface receptors implicated in Alzheimer’s disease
Biophysical Reviews (2022)
-
Deciphering the neuroprotective and neurogenic potential of soluble amyloid precursor protein alpha (sAPPα)
Cellular and Molecular Life Sciences (2020)
-
Regulation of the alternative β-secretase meprin β by ADAM-mediated shedding
Cellular and Molecular Life Sciences (2019)
-
Knockout of Amyloid β Protein Precursor (APP) Expression Alters Synaptogenesis, Neurite Branching and Axonal Morphology of Hippocampal Neurons
Neurochemical Research (2019)