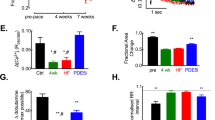
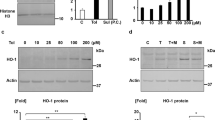
Abstract
The crystal structure of the complex between the binuclear manganese metalloenzyme arginase and the boronic acid analog of L-arginine, 2(S)-amino-6-boronohexanoic acid (ABH), has been determined at 1.7 Å resolution from a crystal perfectly twinned by hemihedry. ABH binds as the tetrahedral boronate anion, with one hydroxyl oxygen symmetrically bridging the binuclear manganese cluster and a second hydroxyl oxygen coordinating to Mn2+A. This binding mode mimics the transition state of a metal-activated hydroxide mechanism. This transition state structure differs from that occurring in NO biosynthesis, thereby explaining why ABH does not inhibit NO synthase. We also show that arginase activity is present in the penis. Accordingly, the tight binding and specificity of ABH allows us to probe the physiological role of arginase in modulating the NO-dependent smooth muscle relaxation required for erection. Strikingly, ABH causes significant enhancement of nonadrenergic, noncholinergic nerve-mediated relaxation of penile corpus cavernosum smooth muscle, suggesting that arginase inhibition sustains L-arginine concentrations for NO synthase activity. Therefore, human penile arginase is a potential target for therapeutic intervention in the treatment of erectile dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Feldman, H. A., Goldstein, I., Hatzichristou, D. G., Krane, R. J & McKinlay, J. B. J. Urol. 151, 54–61 (1994).
Ignarro, L. J. et al. Biochem. Biophys. Res. Commun. 170, 843–850 (1990).
Kim, N., Azadzoi, K. M., Goldstein, I. & Saenz de Tejada, I. J. Clin. Invest. 88, 112–118 (1991).
Burnett, A. L., Chang, T. S. K., Lowenstein, C. J., Bredt, D. S. & Snyder, S. H. Science 257, 401–403 (1992).
Rajfer, J., Aronson, W. J., Bush, P. A., Dorey, F. J. & Ignarro, L. J. New Engl. J. Med. 238, 90–94 (1992).
Burnett, A. L. J. Urol. 157, 320–324 ( 1997).
Reczkowski, R. S. & Ash, D. E. J. Am. Chem. Soc. 114, 10992–10994 ( 1992).
Cavalli, R. C., Burke, C. J., Kawamoto, S., Soprano, D. R. & Ash, D. E. Biochemistry 33, 10652–10657 (1994).
Ash, D. E., Cox, J. D. & Christianson, D. W. In Manganese and its role in biological processes, Vol. 37 of Metal ions in biological systems, Sigel A. & Sigel H., Eds.; New York: M. Dekker, 408–428.
Albina, J. E., Mills, C. D., Henry, W. L. & Caldwell, M. D. J. Immunol. 144, 3877–3880 (1990).
Wang, W. W. et al. Biochem. Biophys. Res. Commun. 210, 1009–1016 (1995).
Chakder, S. & Rattan, S. Am. J. Physiol. 264, G7–G12 (1993).
Chakder, S. & Rattan, S. J. Pharmacol. Exp. Ther. 282, 378–384 (1997).
Baggio, R. et al. J. Pharmacol. Exp. Ther. 290, 1409– 1416 (1999).
Baggio, R. et al. J. Am. Chem. Soc. 119, 8107– 8108 (1997).
Kanyo, Z. F., Scolnick, L. R., Ash, D. E. & Christianson, D. W. Nature 383, 554–557 ( 1996).
Kettner, C. A. & Shenvi, A. B. J. Biol. Chem. 259, 15106–15114 ( 1984).
Shenvi, A. B. Biochemistry 25, 1286–1291 (1986).
Yeates, T. O. Methods Enzymol. 276, 344–358 (1997).
Redinbo, M. R. & Yeates, T. O. Acta Crystallogr. D49, 375–380 ( 1993).
Brünger, A. T. et al. Acta Crystallogr. D54, 905– 921 (1998).
Bewley, M. C., Jeffrey, P. D., Patchett, M. L., Kanyo, Z. F. & Baker, E. N. Structure 7, 435–448 (1999).
Reczkowski, R. S. & Ash, D. E. Arch. Biochem. Biophys. 312, 31–37 ( 1994).
Stuehr, D. J. & Griffith, O. W. Adv. Enzymol. 65, 287–346 (1992).
Saenz de Tejada, I. et al. Am. J. Physiol. 254, H459– H467 (1988).
Moody, J. A., Vernet, D., Laidlaw, S., Rajfer, J. & Gonzalez-Cadavid, N. F. J. Urol. 158, 942– 947 (1997).
Wheeler, M. A., Smith, S. D., Saito, N., Foster, H. E. Jr. & Weiss, R. M. J. Urol. 158, 2045–2050 (1997).
Zorgniotti, A. W. & Lizza, E. F. Int. J. Impotence Res. 6, 33–35 ( 1994).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 ( 1997).
Navaza, J. Acta Crystallogr. A50, 157–163 (1994).
Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Acta. Crystallogr. A47, 110–119 ( 1991).i.
Verdon, C. P., Burton, B. A. & Prior, R. L. Anal. Biochem. 224, 502– 508 (1995).
Rüegg, U. T. & Russell, A. S. Anal. Biochem. 102, 206–212 ( 1980).
Esnouf, R. M. J. Mol. Graphics 15 132–134 (1997).
Merritt, E. A. & Bacon, D. J. Methods Enzymol. 277, 505–524 ( 1997).
Acknowledgements
We thank D. E. Ash, R. Baggio, K. Gauvreau, C. A. Lesburg, R. Marmorstein, M. Redinbo, T. Stams, R. Trievel, and T. Yeates for helpful discussions during the course of this investigation, and we thank P. Adams and A. Brünger for modifications to the refinement program CNS. This work is supported by grants to A.M.T. and D.W.C. from the National Institutes of Health and is based upon research conducted at the Cornell High Energy Synchrotron Source (CHESS) with the use of the Macromolecular Diffraction at CHESS (MacCHESS) facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cox, J., Kim, N., Traish, A. et al. Arginase–boronic acid complex highlights a physiological role in erectile function. Nat Struct Mol Biol 6, 1043–1047 (1999). https://doi.org/10.1038/14929
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/14929
This article is cited by
-
Arginase: shedding light on the mechanisms and opportunities in cardiovascular diseases
Cell Death Discovery (2022)
-
Cryo-EM structures of inhibitory antibodies complexed with arginase 1 provide insight into mechanism of action
Communications Biology (2021)
-
Influence of arginase polymorphisms and arginase levels/activity on the response to erectile dysfunction therapy with sildenafil
The Pharmacogenomics Journal (2018)
-
Serum Arginase II level can be a novel indicator for erectile dysfunction in patients with vasculogenic erectile dysfunction: a comparative study
International Urology and Nephrology (2018)
-
Arginase Structure and Inhibition: Catalytic Site Plasticity Reveals New Modulation Possibilities
Scientific Reports (2017)