Abstract
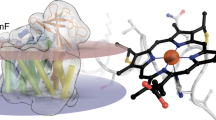
Cytochrome c554 (cyt c554), a tetra-heme cytochrome from Nitrosomonas europaea, is an essential component in the biological nitrification pathway. In N. europaea, ammonia is converted to hydroxylamine, which is then oxidized to nitrite by hydroxylamine oxidoreductase (HAO). Cyt c554 functions in the latter process by accepting pairs of electrons from HAO and transferring them to a cytochrome acceptor. The crystal structure of cyt c554 at 2.6 Å resolution shows a predominantly α-helical protein with four covalently attached hemes. The four hemes are arranged in two pairs such that the planes of the porphyrin rings are almost parallel and overlapping at the edge; corresponding heme arrangements are observed in other multi-heme proteins. Striking structural similarities are evident between the tetra-heme core of cyt c554 and hemes 3–6 of HAO, which suggests an evolutionary relationship between these redox partners.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Andersson, K., Lipscomb, J., Valentine, M., Münck, E. & Hooper, A. Tetraheme cytochrome c-554 from Nitrosomonas europaea: heme-heme interactions and ligand binding. J. Biol. Chem. 261, 1126–1138 (1986).
Yamanaka, T. & Shinra, M. Cytochrome c-552 and cytochrome c-554 derived from Nitrosomonas europaea: purification, properties, and their function in hydroxylamine oxidation. J. Biochem. 75 , 1265–1273 (1974).
Bergmann, D., Arciero, D. & Hooper, A. Organization of the hao gene cluster of Nitrosomonas europaea: genes for two tetraheme c cytochromes. J. Bacteriol. 176, 3148–3153 ( 1994).
Arciero, D., Collins, M., Haladjian, J., Bianco, P. & Hooper, A. Resolution of the four hemes of cytochrome c554 from Nitrosomonas europaea by redox potentiometry and optical spectroscopy. Biochemistry 30, 11459– 11465 (1991).
Arciero, D., Balny, C. & Hooper, A. Spectroscopic and rapid kinetic studies of reduction of cytochrome c554 by hydroxylamine oxidoreductase from Nitrosomonas europaea . Biochemistry 30, 11466– 11472 (1991).
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993).
Matias, P., Frazao, C., Morais, J., Coll, M. & Carrondo, M. Structure analysis of cytochrome c3 from Desulfovibrio vulgaris Hildenborough at 1.9 Å resolution. J Mol. Biol. 234, 680–699 (1993).
Deisenhofer, J., Epp, O., Miki, K., Huber, R. & Michel, H. Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3 Å resolution. Nature 318, 618–624 (1985).
Coutinho, I.B., Turner, D.L., Liu, M.Y., LeGall, J. & Xavier, A.V. Structure of the three-haem core of cytochrome c 551.5 determined by 1H NMR. J. Biol. Inorg. Chem. 1, 305–311 ( 1996).
Igarashi, N., Moriyama, H., Fujiwara, T., Fukumori, Y. & Tanaka, N. The 2.8 Å structure of hydroxlyamine oxidoreductase from a nitrifying chemoautotrophic bacterium, Nitrosomonas europea. Nature Struct. Biol. 4, 276 –284 (1997).
Matias, P. et al. A preliminary analysis of the three-dimensional structure of dimeric di-haem split-Soret cytochrome c from Desulfovibrio desulfuricans ATCC 27774 at 2.5 Å resolution using the MAD phasing method: a novel cytochrome fold with a stacked-haem arrangement. J. Biol. Inorg. Chem. 2, 507–514 (1997).
Degtyarenko, K.N., North, A.C.T., Perkins, D.N. & Findlay, J.B.C. PROMISE: a database of information on prosthetic centres and metal ions in protein active sites. Nucleic Acids Res. 26, 376–381 (1998).
Moore, G. & Pettigrew, G. Cytochromes c—evolutionary, structural and physiochemical aspects (Springer-Verlag, Berlin; 1990).
Loew, G.H. In Iron porphyrins (eds Lever, A.P.B. & Gray, H.B.) (Addison-Wesley Publishing Co., Reading; 1983).
Ludwig, M. et al. Control of oxidation-reduction potentials in flavodoxin from Clostridium beijerinckii: the role of conformational changes. Biochemistry 36, 1259–1280 (1997).
Rees, D.C. & Farrelly, D. Biological electron transfer. The enzymes 19, 37–97 ( 1990).
Stephens, P.J., Jollie, D.R. & Warshel, A. Protein control of redox potential of iron-sulfur proteins. Chem. Rev. 96, 2491–2513 (1996).
Hooper, A., Maxwell, P. & Terry, K. Hydroxylamine oxidoreductase from Nitrosomonas: absorption spectra and content of heme and metal. Biochemistry 17, 2984–2989 ( 1978).
Petsko, G. Preparation of isomorphous heavy-atom derivatives. Meth. Enz. 114, 147–157 (1985).
Holden, H.M. & Rayment, I. Trimethyl lead acetate—a first choice heavy atom derivative for protein crystallography. Arch. Biochem. 291, 187–194 ( 1991).
Nave, C. Radiation damage in protein crystallography. Radiat. Phys. Chem. 45, 483–490 ( 1995).
Gonzales, A. & Nave, C. Radiation damage in protein crystals at low temperature. Acta Crystallgr. D 50, 874–877 (1994).
Otwinowski, Z. In CCP4 study weekend data collection and processing (eds Sawyer, L., Isaacs, N. & Bailey, S.) 56–62 (SERC Daresbury Laboratory, UK; 1993).
Bailey, S. The CCP4 suite—programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Sheldrick, G.M. Phase annealing in SHELX-90—direct methods for larger structures. Acta Crystallogr. A 46, 467–473 (1990).
De La Fortelle, E. & Bricogne, G. Maximum likelihood heavy atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Meth. Enz. 276, 472–494 (1997).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. X-PLOR version 3.1—A system for x-ray crystallography and NMR (Yale University Press, New Haven and London; 1992).
Laskowski, R.A., Mcarthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK - a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283– 291 (1993).
Prince, R.C. & George, G.N. The remarkable complexity of hydroxylamine oxidoreductase. Nature Struct. Biol. 4, 247–250 (1997).
McTavish, H., Arciero, D. & Hooper, A. Interaction with membranes of cytochrome c554 from Nitrosomonas europaea. Arch. Biochem. Biophys. 324, 53–58 (1995).
Kraulis, P.J. MOLSCRIPT—a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallgr. 24, 946–950 (1991).
Esnouf, R. BOBSCRIPT—An extensively modified version of Molscript that includes greatly enhanced coloring capabilities. J. Mol. Graph. 15, 133–138 (1997).
Merritt, E.A. & Murphy, M.E.P. Raster3D Version 2.0—a program for photorealistic molecular graphics. Acta Crystallogr. D50, 869–873 ( 1994).
Sharp, K., Fine, R. & Honig, B. Computer simulations of the diffusion of a substrate to an active site of an enzyme. Science 236, 1460– 1463 (1987).
Allen, F. & Kennard, O. 3D search and research using the Cambridge Data Base. Chemical Design and Automation News 8, 1&31–37 (1993).
Acknowledgements
We thank J. Hu, L. Joshua-Tor, M. Stowell, D. Cascio, M. Soltis, H. Schindelin, C. Kisker and C. Kielkopf for experimental assistance; C. Drennan and H. Axelrod for critical reading and helpful discussions; K. Matthews and B. Crane for the microspectrophotometery; M. Carrondo for the split Soret cytochrome coordinates; and R. Timkovich for the cytochrome c552 coordinates. This work was supported by an NSF grant to A.B.H and an NIH grant to D.C.R. T.M.I. is supported by an NIH fellowship. The rotation camera facility at SSRL is supported by the NIH and Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iverson, T., Arciero, D., Hsu, B. et al. Heme packing motifs revealed by the crystal structure of the tetra-heme cytochrome c554 from Nitrosomonas europaea. Nat Struct Mol Biol 5, 1005–1012 (1998). https://doi.org/10.1038/2975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2975
This article is cited by
-
Nature's nitrite-to-ammonia expressway, with no stop at dinitrogen
JBIC Journal of Biological Inorganic Chemistry (2022)
-
Properties and structure of a low-potential, penta-heme cytochrome c552 from a thermophilic purple sulfur photosynthetic bacterium Thermochromatium tepidum
Photosynthesis Research (2019)
-
Upon further analysis, neither cytochrome c554 from Nitrosomonas europaea nor its F156A variant display NO reductase activity, though both proteins bind nitric oxide reversibly
JBIC Journal of Biological Inorganic Chemistry (2018)
-
Hidden relationships between metalloproteins unveiled by structural comparison of their metal sites
Scientific Reports (2015)
-
The octahaem MccA is a haem c–copper sulfite reductase
Nature (2015)