Abstract
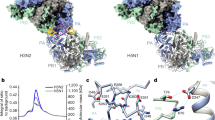
The nonstructural protein NS1 of influenza virus is an antagonist of host immune responses and is implicated in virulence. It has two domains, an N-terminal double-stranded RNA–binding domain (RBD) and an effector domain crucial for RBD function, for nuclear export and for sequestering messenger RNA–processing proteins. Here we present the crystallographic structure of the effector domain, which has a novel fold and suggests mechanisms for increased virulence in H5N1 strains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Lamb, R.A. & Krug, R.M., in Fields Virology Vol. 1, 1487–1531 (Lippincott-Raven, Philadelphia, 2001).
Garcia-Sastre, A. et al. Virology 252, 324–330 (1998).
Wang, X. et al. J. Virol. 74, 11566–11573 (2000).
Krug, R.M., Yuan, W., Noah, D.L. & Latham, A.G. Virology 309, 181–189 (2003).
Lu, Y., Wambach, M., Katze, M.G. & Krug, R.M. Virology 214, 222–228 (1995).
Donelan, N.R., Basler, C.F. & Garcia-Sastre, A. J. Virol. 77, 13257–13266 (2003).
Ferko, B. et al. J. Virol. 78, 13037–13045 (2004).
Greenspan, D., Palese, P. & Krystal, M. J. Virol. 62, 3020–3026 (1988).
Seo, S.H., Hoffmann, E. & Webster, R.G. Nat. Med. 8, 950–954 (2002).
Seo, S.H., Hoffmann, E. & Webster, R.G. Virus Res. 103, 107–113 (2004).
Lipatov, A.S. et al. J. Gen. Virol. 86, 1121–1130 (2005).
Liu, J. et al. Nat. Struct. Biol. 4, 896–899 (1997).
Holm, L. & Park, J. Bioinformatics 16, 566–567 (2000).
Noah, D.L., Twu, K.Y. & Krug, R.M. Virology 307, 386–395 (2003).
Privalsky, M.L. & Penhoet, E.E. Proc. Natl. Acad. Sci. USA 75, 3625–3629 (1978).
Skorko, R., Summers, D.F. & Galarza, J.M. Virology 180, 668–677 (1991).
Wurzer, W.J. et al. EMBO J. 22, 2717–2728 (2003).
Li, W.X. et al. Proc. Natl. Acad. Sci. USA 101, 1350–1355 (2004).
Acknowledgements
We thank P. Palese (Mt. Sinai School of Medicine) for the NS1 clone and P. Wyde for helpful discussions. We acknowledge support from the US National Institutes of Health (AI36040) and the Welch Foundation to B.V.V.P. and from a US National Institutes of Health virology training grant (AI07471) to Z.A.B. We thank H. Bellamy and the staff at the Center for Advanced Microstructures & Devices, as well as the staff of beamline SBC-CAT 19ID at the Advanced Photon Source (supported by the US Department of Energy, Basic Energy Sciences, Office of Science), for their help during data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Size-exclusion chromatography of NS1ΔN73 (PDF 86 kb)
Supplementary Fig. 2
Topology diagram of the monomer. (PDF 123 kb)
Supplementary Fig. 3
Model for the orientation of the RBD in the NS1 protein (PDF 138 kb)
Supplementary Table 1
Data collection and model refinement statistics (PDF 83 kb)
Rights and permissions
About this article
Cite this article
Bornholdt, Z., Prasad, B. X-ray structure of influenza virus NS1 effector domain. Nat Struct Mol Biol 13, 559–560 (2006). https://doi.org/10.1038/nsmb1099
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1099
This article is cited by
-
Major contribution of the RNA-binding domain of NS1 in the pathogenicity and replication potential of an avian H7N1 influenza virus in chickens
Virology Journal (2018)
-
A naturally truncated NS1 protein of influenza A virus impairs its interferon-antagonizing activity and thereby confers attenuation in vitro
Archives of Virology (2017)
-
Mutations associated with severity of the pandemic influenza A(H1N1)pdm09 in humans: a systematic review and meta-analysis of epidemiological evidence
Archives of Virology (2014)
-
Adaptive mutation in influenza A virus non-structural gene is linked to host switching and induces a novel protein by alternative splicing
Emerging Microbes & Infections (2012)
-
Heterologous interactions between NS1 proteins from different influenza A virus subtypes/strains
Science China Life Sciences (2012)