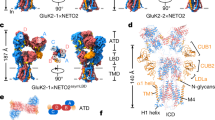
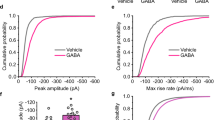
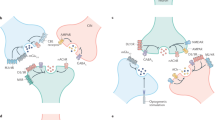
Key Points
-
Kainate receptors are a class of ionotropic glutamate receptors with remarkable structural diversity. There are five different subunits, some of which are susceptible to alternative splicing and mRNA editing. Pharmacologically, they are difficult to distinguish from other glutamate receptors, such as the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) type, but important progress has been made in identifying several relatively selective agonists and antagonists.
-
Kainate receptors have pre- and postsynaptic distributions. Postsynaptic kainate receptors contribute to synaptic transmission, and the slow time-course of their response endows synapses with longer integration times. Presynaptically, kainate receptors modulate transmitter release from excitatory and inhibitory synapses in different brain regions. The presynaptic mechanisms whereby kainate receptors modulate excitation and inhibition are quite complex and remain a matter of debate.
-
Kainate receptors are also involved in synaptic plasticity during development and in the adult, but their mechanism of action remains to be elucidated. Similarly, the long-held view that kainate receptors are relevant to the generation of epilepsy is a matter of debate, as there is contradictory evidence on their role as pro- or anticonvulsant molecules.
-
In addition to these debates in the field, two additional observations make kainate receptors one of the most intriguing receptor family in the nervous system. First, although there are several kainate receptor-knockout animals, they have not been particularly informative about the function of these receptors. Second, some actions of kainate seem to involve the unusual interaction of an ionotropic receptor with a G protein.
Abstract
Functional kainate receptors are ubiquitous in the central nervous system. After a search for their functional significance, a considerable amount of data indicates that this class of glutamate receptors is present at both sides of the synapse. Pre- and postsynaptic kainate receptors can regulate transmission at many synapses in a specific manner, and seem to be involved in short- and long-term plastic phenomena, highlighting their significance for synaptic signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ramón y Cajal, S. Significación fisiológica de las expansiones protoplásmicas y nerviosas de las células de la sustancia gris. Rev. Ciencias Med. Barcelona 22, 1–15 (1881).
Cowan, M., Sudhof, T. C. & Stevens, C. (eds) Synapses. (The Johns Hopkins Univ. Press Baltimore, Massachusetts, 2001).
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 (1994).
Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597–603 (1992).
Seeburg, P. H. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 16, 359–365 (1993).
Paternain, A. V., Morales, M. & Lerma, J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14, 185–189 (1995). The first description that 2,3-benzodiazepine, GYKI 53655, is a specific antagonist of AMPA receptors that unmasks the presence of kainate receptors in hippocampal neurons and can therefore be used to isolate one kainate receptor from the other.
Wilding, T. J. & Huettner, J. E. Differential antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol. Pharmacol. 47, 582–587 (1995). Describes the specificity of GYKI 53655 to antagonize AMPA over kainate receptors.
Ben-Ari, Y. & Cossart, R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 23, 580–587 (2000).
Bleakman, D. Kainate receptor pharmacology and physiology. Cell. Mol. Life Sci. 56, 558–566 (1999).
Chittajallu, R., Braithwaite, S. P., Clarke, V. R. & Henley, J. M. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol. Sci. 20, 26–35 (1999).
Frerking, M. & Nicoll, R. A. Synaptic kainate receptors. Curr. Opin. Neurobiol. 10, 342–351 (2000).
Huettner, J. E. Kainate receptors: knocking out plasticity. Trends Neurosci. 24, 365–366 (2001).
Kamiya, H. Kainate receptor-dependent presynaptic modulation and plasticity. Neurosci. Res. 42, 1–6 (2002).
Lerma, J., Paternain, A. V., Rodriguez-Moreno, A. & López-García, J. C. Molecular physiology of kainate receptors. Physiol. Rev. 81, 971–998 (2001).
Madden, D. R. The structure and function of glutamate receptor ion channels. Nature Rev. Neurosci. 3, 91–101 (2002).
Stern-Bach, Y. et al. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron 13, 1345–1357 (1994).
Swanson, G. T., Gereau, R. W., Green, T. & Heinemann, S. F. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron 19, 913–926 (1997).
Fleck, M. W., Cornell, E. & Mah, S. J. Amino-acid residues involved in glutamate receptor 6 kainate receptor gating and desensitization. J. Neurosci. 23, 1219–1227 (2003).
Bowie, D. External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J. Physiol. (Lond.) 539, 725–733 (2002).
Sommer, B. et al. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 11, 1651–1656 (1992).
Paternain, A. V., Herrera, M. T., Nieto, M. A. & Lerma, J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J. Neurosci. 20, 196–205 (2000).
Schiffer, H. H., Swanson, G. T. & Heinemann, S. F. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron 19, 1141–1146 (1997).
Cui, C. & Mayer, M. L. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J. Neurosci. 19, 8281–8291 (1999).
Bettler, B. et al. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron 5, 583–595 (1990). Cloning of the first kainate receptor subunit.
Gregor, P., O'Hara, B. F., Yang, X. & Uhl, G. R. Expression and novel subunit isoforms of glutamate receptor genes GluR5 and GluR6. Neuroreport 4, 1343–1346 (1993).
Seeburg, P. H. The role of RNA editing in controlling glutamate receptor channel properties. J. Neurochem. 66, 1–5 (1996).
Sommer, B., Kohler, M., Sprengel, R. & Seeburg, P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 (1991).
Swanson, G. T., Feldmeyer, D., Kaneda, M. & Cull-Candy, S. G. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J. Physiol. (Lond). 492, 129–142 (1996).
Kohler, M., Burnashev, N., Sakmann, B. & Seeburg, P. H. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron 10, 491–500 (1993).
Bischoff, S., Barhanin, J., Bettler, B., Mulle, C. & Heinemann, S. Spatial distribution of kainate receptor subunit mRNA in the mouse basal ganglia and ventral mesencephalon. J. Comp. Neurol. 379, 541–562 (1997).
Wisden, W. & Seeburg, P. H. A complex mosaic of high-affinity kainate receptors in rat brain. J. Neurosci. 13, 3582–3598 (1993).
Bahn, S., Volk, B. & Wisden, W. Kainate receptor gene expression in the developing rat brain. J. Neurosci. 14, 5525–5547 (1994).
Brandstatter, J. H., Koulen, P. & Wassle, H. Selective synaptic distribution of kainate receptor subunits in the two plexiform layers of the rat retina. J. Neurosci. 17, 9298–9307 (1997).
Grunert, U., Haverkamp, S., Fletcher, E. L. & Wassle, H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J. Comp. Neurol. 447, 138–151 (2002).
Haverkamp, S., Ghosh, K. K., Hirano, A. A. & Wassle, H. Immunocytochemical description of five bipolar cell types of the mouse retina. J. Comp. Neurol. 455, 463–476 (2003).
Haverkamp, S., Grunert, U. & Wassle, H. Localization of kainate receptors at the cone pedicles of the primate retina. J. Comp. Neurol. 436, 471–486 (2001).
Petralia, R. S., Wang, Y. X. & Wenthold, R. J. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J. Comp. Neurol. 349, 85–110 (1994).
Charara, A., Blankstein, E. & Smith, Y. Presynaptic kainate receptors in the monkey striatum. Neuroscience 91, 1195–1200 (1999).
Kieval, J. Z., Hubert, G. W., Charara, A., Pare, J. F. & Smith, Y. Subcellular and subsynaptic localization of presynaptic and postsynaptic kainate receptor subunits in the monkey striatum. J. Neurosci. 21, 8746–8757 (2001).
Bureau, I., Dieudonne, S., Coussen, F. & Mulle, C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc. Natl Acad. Sci. USA 97, 6838–6843 (2000).
Castillo, P. E., Malenka, R. C. & Nicoll, R. A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997). Together with reference 48, this is the first description of synaptic activation of kainate receptors.
Cossart, R., Esclapez, M., Hirsch, J. C., Bernard, C. & Ben-Ari, Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nature Neurosci. 1, 470–478 (1998).
DeVries, S. H. & Schwartz, E. A. Kainate receptors mediate synaptic transmission between cones and 'Off' bipolar cells in a mammalian retina. Nature 397, 157–160 (1999).
Frerking, M., Malenka, R. C. & Nicoll, R. A. Synaptic activation of kainate receptors on hippocampal interneurons. Nature Neurosci. 1, 479–486 (1998).
Kidd, F. L. & Isaac, J. T. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400, 569–573 (1999). Describes the presence of kainate receptors at thalamocortical synapses, showing a developmental conversion of transmission from being predominantly mediated by kainate receptors to depend primarily on AMPA receptors.
Li, H. & Rogawski, M. A. GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology 37, 1279–1286 (1998).
Li, P. et al. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 397, 161–164 (1999).
Vignes, M. & Collingridge, G. L. The synaptic activation of kainate receptors. Nature 388, 179–182 (1997).
Lerma, J. Kainate reveals its targets. Neuron 19, 1155–1158 (1997).
Kidd, F. L. & Isaac, J. T. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J. Neurophysiol. 86, 1139–1148 (2001).
Cossart, R. et al. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35, 147–159 (2002). Reports that, in hippocampal neurons, the quantal release of glutamate generates kainate receptor-mediated EPSCs that provide half of the total synaptic current.
Nadal, M. S. et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 37, 449–461 (2003).
Garcia, E. P. et al. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron 21, 727–739 (1998).
Coussen, F. et al. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J. Neurosci. 22, 6426–6436 (2002).
Hirbec, H. et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37, 625–638 (2003). Describes rapid regulation of postsynaptic kainate receptors by association with GRIP and PICK1.
Raymond, L. A., Blackstone, C. D. & Huganir, R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature 361, 637–641 (1993).
Traynelis, S. F. & Wahl, P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J. Physiol. (Lond.) 503, 513–531 (1997).
Frerking, M. & Ohliger-Frerking, P. AMPA receptors and kainate receptors encode different features of afferent activity. J. Neurosci. 22, 7434–7443 (2002).
DeVries, S. H. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28, 847–856 (2000).
Paternain, A. V., Rodriguez-Moreno, A., Villarroel, A. & Lerma, J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology 37, 1249–1259 (1998).
Agrawal, S. G. & Evans, R. H. The primary afferent depolarizing action of kainate in the rat. Br. J. Pharmacol. 87, 345–355 (1986).
Represa, A., Tremblay, E. & Ben-Ari, Y. Kainate binding sites in the hippocampal mossy fibers: localization and plasticity. Neuroscience 20, 739–748 (1987).
Monaghan, D. T. & Cotman, C. W. The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 252, 91–100 (1982).
Contractor, A., Swanson, G. T., Sailer, A., O'Gorman, S. & Heinemann, S. F. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J. Neurosci. 20, 8269–8278 (2000).
Lauri, S. E. et al. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron 32, 697–709 (2001). Together with reference 66, this paper illustrates the role for kainate autoreceptors in the bidirectional modulation of glutamate release at mossy fibre synapses and in the generation of LTP.
Schmitz, D., Mellor, J. & Nicoll, R. A. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science 291, 1972–1976 (2001).
Schmitz, D., Frerking, M. & Nicoll, R. A. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron 27, 327–338 (2000).
Schmitz, D., Mellor, J., Frerking, M. & Nicoll, R. A. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc. Natl Acad. Sci. USA 98, 11003–11008 (2001).
Kamiya, H. & Ozawa, S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J. Physiol. (Lond.) 523, 653–665 (2000).
Kamiya, H., Ozawa, S. & Manabe, T. Kainate receptor-dependent short-term plasticity of presynaptic Ca2+ influx at the hippocampal mossy fiber synapses. J. Neurosci. 22, 9237–9243 (2002).
Andersen, P., Silfvenius, H., Sundberg, S. H., Sveen, O. & Wigstrom, H. Functional characteristics of unmyelinated fibres in the hippocampal cortex. Brain Res. 144, 11–18 (1978).
Henze, D. A., Urban, N. N. & Barrionuevo, G. Origin of the apparent asynchronous activity of hippocampal mossy fibers. J. Neurophysiol. 78, 24–30 (1997).
Semyanov, A. & Kullmann, D. M. Kainate receptor-dependent axonal depolarization and action potential initiation in interneurons. Nature Neurosci. 4, 718–723 (2001).
Henze, D. A., Urban, N. N. & Barrionuevo, G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98, 407–427 (2000).
Perkinton, M. S. & Sihra, T. S. A high-affinity presynaptic kainate-type glutamate receptor facilitates glutamate exocytosis from cerebral cortex nerve terminals (synaptosomes). Neuroscience 90, 1281–1292 (1999).
Geiger, J. R. & Jonas, P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron 28, 927–39 (2000).
Delaney, A. J. & Jahr, C. E. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron 36, 475–482 (2002). Reports a differential frequency-dependent sensitivity of two synapses: low-frequency stimulation of parallel fibres facilitates glutamate release onto both stellate and Purkinje cells, whereas high-frequency stimulation depresses release onto stellate cell but still facilitates Purkinje cell synapses.
Chittajallu, R. et al. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379, 78–81 (1996). First paper describing an action of kainate receptors on glutamate release in the hippocampus.
Vignes, M. et al. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology 37, 1269–1277 (1998).
Kamiya, H. & Ozawa, S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J. Physiol. (Lond.) 509, 833–845 (1998).
Frerking, M., Schmitz, D., Zhou, Q., Johansen, J. & Nicoll, R. A. Kainate receptors depress excitatory synaptic transmission at CA3-CA1 synapses in the hippocampus via a direct presynaptic action. J. Neurosci. 21, 2958–2966 (2001).
Cunha, R., Malva, J. O. & Ribeiro, J. A. Pertussis toxin prevents presynaptic inhibition by kainate receptors of rat hippocampal [3H]GABA release. FEBS Lett. 469, 159–162 (2000).
Rodriguez-Moreno, A. & Lerma, J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218 (1998). Describes that modulation of GABA release by kainate receptors is sensitive to Pertussis toxin and inhibitors of PKC, pointing, for the first time, to a metabotropic role in kainate receptor-mediated signalling.
Rodriguez-Moreno, A., López-García, J. C. & Lerma, J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc. Natl Acad. Sci. USA 97, 1293–1298 (2000).
Contractor, A., Swanson, G. & Heinemann, S. F. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron 29, 209–216 (2001).
Bortolotto, Z. A. et al. Kainate receptors are involved in synaptic plasticity. Nature 402, 297–301 (1999). First indication that kainate receptors are required for induction of LTP at CA3 synapses.
Contractor, A. et al. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J. Neurosci. 23, 422–429 (2003).
Sloviter, R. S. & Damiano, B. P. On the relationship between kainic acid-induced epileptiform activity and hippocampal neuronal damage. Neuropharmacology 20, 1003–1011 (1981).
Westbrook, G. L. & Lothman, E. W. Cellular and synaptic basis of kainic acid-induced hippocampal epileptiform activity. Brain Res. 273, 97–109 (1983).
Fisher, R. S. & Alger, B. E. Electrophysiological mechanisms of kainic acid-induced epileptiform activity in the rat hippocampal slice. J. Neurosci. 4, 1312–1323 (1984).
Kehl, S. J. & McLennan, H. An electrophysiological characterization of inhibitions and postsynaptic potentials in rat hippocampal CA3 neurones in vitro. Exp. Brain Res. 60, 299–308 (1985).
Clarke, V. R. et al. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389, 599–603 (1997).
Rodriguez-Moreno, A., Herreras, O. & Lerma, J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901 (1997). This paper and reference 93 describe that kainate receptor activation depresses GABA-mediated transmission through a presynaptic mechanism.
Frerking, M., Petersen, C. C. & Nicoll, R. A. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc. Natl Acad. Sci. USA 96, 12917–12922 (1999).
Mulle, C. et al. Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28, 475–484 (2000).
Ali, A. B., Rossier, J., Staiger, J. F. & Audinat, E. Kainate receptors regulate unitary IPSCs elicited in pyramidal cells by fast-spiking interneurons in the neocortex. J. Neurosci. 21, 2992–2999 (2001).
Cossart, R. et al. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29, 497–508 (2001).
Freund, T. F. & Buzsaki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Jiang, L., Xu, J., Nedergaard, M. & Kang, J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron 30, 503–513 (2001).
Braga, M. F., Aroniadou-Anderjaska, V., Xie, J. & Li, H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J. Neurosci. 23, 442–452 (2003).
Liu, Q. S., Patrylo, P. R., Gao, X. B. & van den Pol, A. N. Kainate acts at presynaptic receptors to increase GABA release from hypothalamic neurons. J. Neurophysiol. 82, 1059–62 (1999).
Kerchner, G. A., Wang, G. D., Qiu, C. S., Huettner, J. E. & Zhuo, M. Direct presynaptic regulation of GABA/glycine release by kainate receptors in the dorsal horn. An ionotropic mechanism. Neuron 32, 477–488 (2001).
Kullmann, D. M., Erdemli, G. & Asztely, F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron 17, 461–474 (1996).
Min, M. Y., Melyan, Z. & Kullmann, D. M. Synaptically released glutamate reduces γ-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proc. Natl Acad. Sci. USA 96, 9932–9937 (1999). Examines the effect of synaptically released glutamate on monosynaptic inhibitory signalling. Describes that brief bursts of activity in glutamatergic afferent fibres reduce GABA-mediated transmission. Concludes that the net kainate receptor-mediated effect of synaptically released glutamate is to reduce monosynaptic inhibition.
Weisskopf, M. G. & Nicoll, R. A. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature 376, 256–259 (1995).
Yeckel, M. F., Kapur, A. & Johnston, D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nature Neurosci. 2, 625–633 (1999).
Bureau, I., Bischoff, S., Heinemann, S. F. & Mulle, C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J. Neurosci. 19, 653–663 (1999).
Isaac, J. T., Nicoll, R. A. & Malenka, R. C. Evidence for silent synapses: implications for the expression of LTP. Neuron 15, 427–434 (1995).
Daw, M. I. et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28, 873–886 (2000).
Li, H., Chen, A., Xing, G., Wei, M. L. & Rogawski, M. A. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nature Neurosci. 4, 612–620 (2001).
Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14, 375–403 (1985).
Cossart, R. et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nature Neurosci. 4, 52–62 (2001).
Khalilov, I., Hirsch, J., Cossart, R. & Ben-Ari, Y. Paradoxical anti-epileptic effects of a GluR5 agonist of kainate receptors. J. Neurophysiol. 88, 523–527 (2002).
Smolders, I. et al. Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nature Neurosci. 5, 796–804 (2002). Reports that selective antagonists of GluR5-containing kainate receptors prevent the development of epileptiform activity induced by pilocarpine and inhibited pre-established epileptic activity.
Huettner, J. E. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron 5, 255–266 (1990). First description of a class of glutamate receptors with differential sensitivity to kainate and domoate, the activation of which generated a transient response.
Lerma, J., Paternain, A. V., Naranjo, J. R. & Mellstrom, B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc. Natl Acad. Sci. USA 90, 11688–11692 (1993). Provided the first functional evidence that there are pure kainate receptors in central neurons.
Jones, K. A., Wilding, T. J., Huettner, J. E. & Costa, A. M. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology 36, 853–863 (1997).
Verdoorn, T. A., Johansen, T. H., Drejer, J. & Nielsen, E. O. Selective block of recombinant glur6 receptors by NS-102, a novel non-NMDA receptor antagonist. Eur. J. Pharmacol. 269, 43–49 (1994).
Simmons, R. M. et al. Kainate GluR5 receptor subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology 37, 25–36 (1998).
Bleakman, D. et al. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology 35, 1689–1702 (1996).
O'Neill, M. J. et al. LY377770, a novel iGluR5 kainate receptor antagonist with neuroprotective effects in global and focal cerebral ischaemia. Neuropharmacology 39, 1575–88 (2000).
Huettner, J. E., Stack, E. & Wilding, T. J. Antagonism of neuronal kainate receptors by lanthanum and gadolinium. Neuropharmacology 37, 1239–1247 (1998).
Melyan, Z., Wheal, H. V. & Lancaster, B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34, 107–114 (2002).
Wang, Y., Small, D. L., Stanimirovic, D. B., Morley, P. & Durkin, J. P. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature 389, 502–504 (1997).
Kawai, F. & Sterling, P. AMPA receptor activates a G-protein that suppresses a cGMP-gated current. J. Neurosci. 19, 2954–2959 (1999).
Mulle, C. et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392, 601–605 (1998). Describes that CA3 hippocampal neurons of GluR6 mutant mice are less sensitive to kainate. Shows that the slow postsynaptic kainate receptor-mediated current in CA3 neurons evoked by stimulation of the mossy fibre is absent in the mutant and that these mice are less susceptible to develop seizures upon systemic administration of kainate.
Kerchner, G. A., Wilding, T. J., Huettner, J. E. & Zhuo, M. Kainate receptor subunits underlying presynaptic regulation of transmitter release in the dorsal horn. J. Neurosci. 22, 8010–8017 (2002).
Sailer, A. et al. Generation and analysis of GluR5(Q636R) kainate receptor mutant mice. J. Neurosci. 19, 8757–8764 (1999).
Vissel, B. et al. The role of RNA editing of kainate receptors in synaptic plasticity and seizures. Neuron 29, 217–227 (2001). Indicates that editing of the GluR6 Q/R site modulates synaptic plasticity and seizure vulnerability.
Egebjerg, J., Bettler, B., Hermans-Borgmeyer, I. & Heinemann, S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature 351, 745–748 (1991). Cloning of the GluR6 subunit.
Bettler, B. et al. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron 8, 257–65 (1992).
Werner, P., Voigt, M., Keinänen, K., Wisden, W. & Seeburg, P. H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature 351, 742–744 (1991).
Herb, A. et al. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron 8, 775–785 (1992).
Acknowledgements
I thank my present (O. Herreras, K. Jones, A. V. Paternain, J. L. Rozas) and former (J. C. López, A. Rodriguez-Moreno) collaborators for discussion, as well as D. Guinea for technical assistance. I thank R. Gallego (Universidad Miguel Hernández) for critical reading of the manuscript. Work in my laboratory has been supported by grants from the Spanish Ministry of Science and Technology, the Community of Madrid and the European Commission.
Author information
Authors and Affiliations
Glossary
- EC50/IC50
-
The concentration of an agonist or antagonist that evokes a half-maximal activation (EC50) or inhibition (IC50).
- ALTERNATIVE SPLICING
-
During splicing, introns are excised from RNA after transcription and the cut ends are rejoined to form a continuous message. Alternative splicing gives rise to different messages from the same DNA molecule.
- mRNA EDITING
-
Site-specific change in an RNA sequence that results in RNA anticodons, which differ from that designated by their DNA templates. In glutamate receptors, the most common change is the conversion of adenosine to inosine.
- RECTIFICATION
-
The property whereby current through a channel does not flow with the same ease from the inside as from the outside. In inward rectification, for instance, current into the cell flows more easily than out of the cell through the same population of channels.
- IN SITU HYBRIDIZATION
-
Method used to label cells or chromosomes having specific sequences of nucleic acids. Particularly used to identify mRNA expression in cytohistological preparations, this technique detects the formation of nucleic acid hybrid molecules between the target nucleic acid and the labelled probe containing a complementary sequence.
- PARALLEL FIBRES
-
Axons of cerebellar granule cells. Parallel fibres emerge from the molecular layer of the cerebellar cortex towards the periphery, where they extend branches perpendicular to the main axis of the Purkinje neurons and form the so-called en passant synapses with this cell type.
- MOSSY FIBRES
-
Axons of granule cells, which form synapses with CA3 pyramidal neurons. Mossy fibre boutons are among the largest in the central nervous system.
- SCHAFFER COLLATERALS
-
Axons of the CA3 pyramidal cells of the hippocampus that form synapses with the apical dendrites of CA1 neurons.
- QUANTAL RELEASE
-
The release of transmitter from a single synaptic vesicle.
- CHARGE TRANSFER
-
A measure of the magnitude of ion flow through a channel.
- SPIKE THRESHOLD
-
The critical value of membrane potential at which a neurone or an axon will fire an action potential (spike).
- STRATUM LUCIDUM
-
The site of termination of the mossy fibres from the dentate gyrus onto CA3 neurons of the hippocampus.
- AFFERENT VOLLEY
-
The wave of a synaptic field potential with the shortest latency, which is proportional to the number of active presynaptic fibres. Its amplitude serves to estimate the strength of afferent input.
- ELECTRICAL SHUNTING
-
A phenomenon by which membrane depolarization that is induced by a given current is attenuated because of an enhanced membrane conductance.
- ANTIDROMIC
-
An action potential travelling from the axon terminal towards the cell body is said to be antidromic.
- HILUS
-
A subdivision of the hippocampus that is rich in interneurons. It is located between the CA3 region and the dentate gyrus.
- SYNAPTOSOMES
-
Vesicles formed by a synaptic terminal generated after homogenization of nerve tissue. They preserve neurotransmitter-containing vesicles, the main machinery of release, and presynaptic receptors and channels.
- PERTUSSIS TOXIN
-
The causative agent of whooping cough, pertussis toxin causes the persistent activation of Gi proteins by catalysing the ADP-ribosylation of the α-subunit.
- MINIATURE INIHIBITORY POSTSYNAPTIC CURRENTS
-
Synaptic currents observed in the absence of presynaptic action potentials; they are thought to correspond to the response elicited by a single vesicle of transmitter.
- STRATUM ORIENS
-
Hippocampal layer that harbours the basal dendrites of the principal cells.
- ASSOCIATIONAL/COMMISSURAL PATHWAY
-
The projections from CA3 neurons to other CA3 cells on the same (associational) or the opposite (commissural) side of the brain.
- PHASE-LOCKED
-
Neurons that fire preferentially at a certain phase of an amplitude-modulated stimulus.
- LONG-TERM POTENTIATION
-
A long-lasting increase in the efficacy of neurotransmission, which can be elicited by diverse patterns of synaptic activation.
- SILENT SYNAPSE
-
A synapse that contains NMDA receptors but no AMPA receptors and is therefore functionally silent during low-frequency, basal synaptic transmission.
- YEAST TWO-HYBRID SCREENS
-
System used to determine the existence of direct interactions between proteins. It involves the use of plasmids that encode two hybrid proteins; one of them is fused to the GAL4 DNA-binding domain and the other one is fused to the GAL4 activation domain. The two proteins are expressed together in yeast and, if they interact, then the resulting complex will drive the expression of a reporter gene, commonly β-galactosidase.
- PDZ DOMAIN
-
A peptide-binding domain that is important for the organization of membrane proteins, particularly at cell–cell junctions, including synapses. It can bind to the carboxyl termini of proteins or can form dimers with other PDZ domains. PDZ domains are named after the proteins in which these sequence motifs were originally identified (PSD95, Discs large, zona occludens 1).
Rights and permissions
About this article
Cite this article
Lerma, J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4, 481–495 (2003). https://doi.org/10.1038/nrn1118
Issue Date:
DOI: https://doi.org/10.1038/nrn1118
This article is cited by
-
Behavioral analysis of kainate receptor KO mice and the role of GluK3 subunit in anxiety
Scientific Reports (2024)
-
The kainate receptor GluK2 mediates cold sensing in mice
Nature Neuroscience (2024)
-
Aryl Hydrocarbon Receptor in Glia Cells: A Plausible Glutamatergic Neurotransmission Orchestrator
Neurotoxicity Research (2023)
-
Photoswitching fingerprint analysis bypasses the 10-nm resolution barrier
Nature Methods (2022)
-
Receptor architecture of macaque and human early visual areas: not equal, but comparable
Brain Structure and Function (2022)