Key Points
-
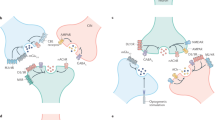
Synaptic targeting and clustering of GABA (γ-aminobutyric acid)and glycine receptors is mediated by the interaction of these receptor subunits with the cytoskeleton. The intracellular domains of individual receptor subunits can interact with several proteins, including cytoskeletal elements, microtubule-binding proteins, neurotransmitter transporters, protein kinases, kinase-anchoring proteins and other signalling molecules. The roles of these protein–protein interactions in the synaptic accumulation and functional modulation of GABAA and glycine receptors have begun to be unravelled and constitute the focus of this review.
-
The large number of GABA and glycine receptor subunits is responsible for the extensive heterogeneity of glycine and GABAA receptor structure. In the case of GABA receptors, the presence of specific subunits in a given receptor subtype can determine receptor trafficking and subcellular localization.
-
Several accessory proteins that facilitate the accumulation of GABAA and glycine receptors at synaptic sites have been identified. In the case of glycine receptors, gephyrin is crucial for their clustering at synapses. Gephyrin can interact with several signalling proteins such as collybistin (a GDP–GTP exchange factor) and Raft1 (a protein involved in the control of translation). This raises the possibility that the action of gephyrin could also involve signal transduction and/or structural remodelling. However, the presence of these proteins at glycine synapses has yet to be definitively proven.
-
There are at least two mechanisms for the synaptic clustering of these GABAA receptors, one dependent and one independent on gephyrin. The components of the gephyrin-independent mechanism have remained elusive but dystrophin has been identified as one possible candidate. A protein known as GABARAP (GABAA receptor-associated protein)can also interact with GABAA receptor γ2 subunits. However, GABARAP is unlikely to have a role in synaptic clustering and might instead be relevant for intracellular transport.
-
GABAC receptors interact both with MAP1B, a molecule capable of binding actin and tubulin, and Glyt1E/F, a novel variant of the glycine transporter. The selective binding of MAP1B to GABAC but not to GABAA receptors could help to explain the differential localization of these receptors in the retina.
-
The dynamic regulation of inhibitory transmitter receptors has begun to be elucidated. GABAA receptors undergo constitutive endocytosis and travel between synaptic sites and endosomal structures but the relevance of this process for synaptic inhibition remains unknown. GABAA receptors are also phosphorylated by several protein kinases and can directly bind both protein kinase C (PKC) and PKC-anchoring proteins. PKC can phosphorylate individual β subunits and thereby modulate GABAA receptor function.
Abstract
Control of nerve-cell excitability is crucial for normal brain function. Two main groups of inhibitory neurotransmitter receptors — GABAA and glycine receptors — fulfil a significant part of this role. To mediate fast synaptic inhibition effectively, these receptors need to be localized and affixed opposite nerve terminals that release the appropriate neurotransmitter at multiple sites on postsynaptic neurons. But for this to occur, neurons require intracellular anchoring molecules, as well as mechanisms that ensure the efficient turnover and transport of mature, functional inhibitory synaptic receptor proteins. This review describes the dynamic regulation of synaptic GABAA and glycine receptors and discusses recent advances in this rapidly evolving field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schofield, P. R. et al. Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature 328, 221–227 (1987).
Grenningloh, G. et al. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 328, 215–220 (1987).
Maricq, A. V., Peterson, A. S., Brake, A. J., Myers, R. M. & Julius, D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254, 432–437 ( 1991).
Julius, D. Molecular biology of serotonin receptors. Annu. Rev. Neurosci. 14, 335–360 ( 1991).
Unwin, N. The nicotinic acetylcholine receptor of the Torpedo electric ray. J. Struct. Biol. 121, 181–190 (1998).
Grenningloh, G. et al. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 9, 771–776 (1990).
Betz, H. et al. Structure and functions of inhibitory and excitatory glycine receptors . Ann. NY Acad. Sci. 868, 667– 676 (1999).
Harvey, R. J. & Betz, H. in Pharmacology of Ionic Channel Function: Activators and Inhibitors (eds Endo, M., Kurachi, Y. & Mishina, M.) 479–497 (Springer–Verlag, Berlin, 2000).
Kuhse, J. et al. Alternative splicing generates two isoforms of the α2 subunit of the inhibitory glycine receptor. FEBS Lett. 283, 73–77 (1991).
Langosch, D., Thomas, L. & Betz, H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl Acad. Sci. USA 85, 7394–7398 ( 1988).
Becker, C. M., Hoch, W. & Betz, H. Glycine receptor heterogeneity in rat spinal cord during postnatal development . EMBO J. 7, 3717–3726 (1988).
Bormann, J. & Feigenspan, A. GABAC receptors. Trends Neurosci. 18, 515–519 (1995).
Bormann, J. The 'ABC' of GABA receptors. Trends Pharmacol. Sci. 21, 16–19 (2000).
Sieghart, W. Unraveling the function of GABAA receptor subtypes. Trends Pharmacol. Sci. 21, 411–413 (2000).
Rabow, L. E., Russek, S. J. & Farb, D. H. From ion currents to genomic analysis: Recent advances in GABAA receptor research. Synapse 21, 189–274 (1995).
Davies, P. A., Hanna, M. C., Hales, T. G. & Kirkness, E. F. Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature 385, 820– 823 (1997).
Hedblom, E. & Kirkness, E. F. A novel class of GABAA receptor subunit in tissues of the reproductive system. J. Biol. Chem. 272, 15346–15350 (1997).
Bonnert, T. P. et al. θ, a novel γ-aminobutyric acid type A receptor subunit. Proc. Natl Acad. Sci. USA 96, 9891 –9896 (1999).
Laurie, D. J., Wisden, W. & Seeburg, P. H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 12, 4151–4172 (1992).
Fritschy, J.-M. & Mohler, H. GABAA receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194 (1995).
Connolly, C. N., Krishek, B. J., McDonald, B. J., Smart, T. G. & Moss, S. J. Assembly and cell surface expression of heteromeric and homomeric γ-aminobutyric acid type A receptors. J. Biol. Chem. 271, 89–96 (1996).
Gorrie, G. H. et al. Assembly of GABAA receptors composed of α1 and β2 subunits in both cultured neurons and fibroblasts. J. Neurosci. 17, 6587–6596 (1997).
Taylor, P. M. et al. Identification of amino acid residues within GABAA receptor β subunits that mediate both homomeric and heteromeric receptor expression. J. Neurosci. 19, 6360– 6371 (1999).
Taylor, P. M. et al. Identification of residues within GABA(A) receptor α subunits that mediate specific assembly with receptor β subunits. J. Neurosci. 20, 1297–1306 (2000).
Sigel, E., Baur, R., Malherbe, P. & Mohler, H. The rat β 1-subunit of the GABAA receptor forms a picrotoxin- sensitive anion channel open in the absence of GABA. FEBS Lett. 257, 377–379 (1989).
Krishek, B. J., Moss, S. J. & Smart, T. G. Homomeric β1 γ-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Mol. Pharmacol. 49, 494– 504 (1996).
Wooltorton, J. R., Moss, S. J. & Smart, T. G. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur. J. Neurosci. 9, 2225–2235 ( 1997).
Connolly, C. N. et al. Subcellular localization and endocytosis of homomeric γ2 subunit splice variants of γ-aminobutyric acid type A receptors. Mol. Cell. Neurosci. 13, 259–271 (1999).
Sanna, E., Garau, F. & Harris, R. A. Novel properties of homomeric β1 γ-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital . Mol. Pharmacol. 47, 213– 217 (1995).
Pritchett, D. B. et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 338, 582–585 (1989).
Angelotti, T. P. & MacDonald, R. L. Assembly of GABAA receptor subunits: α1 β1 and α1 β1 γ2S subunits produce unique ion channels with dissimilar single- channel properties. J. Neurosci. 13, 1429–1440 (1993).
Chang, Y., Wang, R., Barot, S. & Weiss, D. S. Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 16, 5415–5424 (1996).
Tretter, V., Ehya, N., Fuchs, K. & Sieghart, W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737 (1997).
Farrar, S. J., Whiting, P. J., Bonnert, T. P. & McKernan, R. M. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J. Biol. Chem. 274, 10100– 10104 (1999).
Shivers, B. D. et al. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron 3, 327– 337 (1989).
Saxena, N. C. & MacDonald, R. L. Assembly of GABAA receptor subunits: role of the δ subunit. J. Neurosci. 14, 7077–7086 (1994).
Whiting, P. J. et al. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties. J. Neurosci. 17, 5027– 6037 (1997).
Rudolph, U. et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401, 796–800 (1999).
Low, K. et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290, 131– 134 (2000).
McKernan, R. M. et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nature Neurosci. 3, 587–592 (2000).
Nusser, Z., Sieghart, W., Benke, D., Fritschy, J.-M. & Somogyi, P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc. Natl Acad. Sci. USA 93, 11939– 11944 (1996).
Fritschy, J. M., Johnson, D. K., Mohler, H. & Rudolph, U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo . Neurosci. Lett. 249, 99– 102 (1998).
Jones, A. et al. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J. Neurosci. 17, 1350– 1362 (1997).
Nusser, Z., Sieghart, W. & Somogyi, P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 18, 1693–1703 (1998).
Sassoe-Pognetto, M., Panzanelli, P., Sieghart, W. & Fritschy, J. M. Co-localization of multiple GABAA receptor subtypes with gephyrin at postsynaptic sites. J. Comp Neurol. 420, 481–498 (2000).The colocalization of gephyrin and GABA A -receptor immunoreactivity was examined at both the light and electron microscopic levels with antibodies selective for α1–3 and γ2 subunits. The authors showed a consistent colocalization for these receptor subunits and gephyrin in several brain regions, including cerebellum, hippocampus, thalamus and olfactory bulb.
Brickley, S. G., Cull-Candy, S. G. & Farrant, M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes . J. Neurosci. 19, 2960– 2973 (1999).
Dotti, C. G., Parton, R. G. & Simons, K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature 349, 158– 161 (1991).
Jareb, M. & Banker, G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron 20, 855–867 ( 1998).
Connolly, C. N., Wooltorton, J. R., Smart, T. G. & Moss, S. J. Subcellular localization of γ-aminobutyric acid type A receptors is determined by receptor β-subunits. Proc. Natl Acad. Sci. USA 93, 9899–9904 ( 1996).
Cutting, G. R. et al. Cloning of the γ-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl Acad. Sci. USA 88, 2673–2677 (1991).
Lukasiewicz, P. D. GABAC receptors in the vertebrate retina. Mol. Neurobiol. 12, 181–194 ( 1996).
Hackam, A. S., Wang, T.-L., Guggino, W. B. & Cutting, G. R. Sequences in the amino termini of GABAρ and GABAA subunits specify their selective interaction in vitro. J. Neurochem. 70, 40–46 ( 1998).
Hackam, A. S., Guggino, W. B. & Cutting, G. R. The N-terminal domain of human GABA receptor p1 subunits contains signals for homooligomeric and heterooligomeric interaction . J. Biol. Chem. 272, 13750– 13757 (1997).
Qian, H. & Ripps, H. Response kinetics and pharmacological properties of heteromeric receptors formed by coassembly of GABA ρ- and γ 2-subunits. Proc. R. Soc. Lond. B 266, 2419–2425 (1999).
Pan, Z. H., Zhang, D., Zhang, X. & Lipton, S. A. Evidence for coassembly of mutant GABAC ρ1 with GABAA γ2S, glycine α1 and glycine α2 receptor subunits in vitro. Eur. J. Neurosci. 12, 3137–3145 (2000).
Koulen, P., Brandstatter, J. H., Enz, R., Bormann, J. & Wassle, H. Synaptic clustering of GABA(C) receptor rho-subunits in the rat retina. Eur. J. Neurosci. 10 , 115–127 (1998).
Koulen, P. et al. Immunocytochemical localization of the GABAC receptor rho subunits in the cat, goldfish, and chicken retina. J. Comp. Neurol. 380, 520–532 ( 1997).
Schmitt, B., Knaus, P., Becker, C. M. & Betz, H. The M r 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry 26, 805–811 (1987).
Johnson, J. L. & Wadman, S. K. in The Metabolic Basis of Inherited Diseases (eds Scrivner, C. R., Beaudef, A. L., Sly, W. S. & Valle, D.) 2271–2283 (McGraw–Hill, New York, 2001).
Prior, P. et al. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron 8, 1161–1170 (1992).
Ramming, M. et al. Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor-synthesizing and cytoskeleton-associated proteins. Proc. Natl Acad. Sci. USA 97, 10266–10271 ( 2000).
Kirsch, J. & Betz, H. Widespread expression of gephyrin, a putative glycine receptor-tubulin linker protein, in rat brain. Brain Res. 621, 301–310 (1993).
Meyer, G., Kirsch, J., Betz, H. & Langosch, D. Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron 15, 563–572 ( 1995).Using a mutagenic approach, a specific 20-amino-acid motif unique to the glycine receptor β-subunit was identified to mediate binding to gephyrin. This motif is absent from all glycine receptor α-subunits and also GABA A receptor subunits.
Kins, S., Kuhse, J., Laube, B., Betz, H. & Kirsch, J. Incorporation of a gephyrin-binding motif targets NMDA receptors to gephyrin-rich domains in HEK 293 cells. Eur. J. Neurosci. 11, 740–744 ( 1999).
Kneussel, M., Hermann, A., Kirsch, J. & Betz, H. Hydrophobic interactions mediate binding of the glycine receptor β-subunit to gephyrin. J. Neurochem. 72, 1323–1326 (1999).
Kirsch, J., Kuhse, J. & Betz, H. Targeting of glycine receptor subunits to gephryin-rich domains in transfected human embryonic kidney cells. Mol. Cell. Neurosci. 6, 450–461 (1995).
Kirsch, J. et al. The 93-kDa glycine receptor-associated protein binds to tubulin . J. Biol. Chem. 266, 22242– 22245 (1991).
Kirsch, J., Wolters, I., Triller, A. & Betz, H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature 366, 745–748 ( 1993).
Feng, G. et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282, 1321–1324 (1998). Production of a gephryin knockout mouse. The phenotype is lethal and the mouse shows an exaggerated startle response and loss of glycine receptor clustering. In addition, there are gross metabolic deficits in agreement with a role for gephyrin in the production of the Moco cofactor essential for the function of molybdenum-containing enzymes.
Sabatini, D. M. et al. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science 284, 1161– 1164 (1999).
Kins, S., Betz, H. & Kirsch, J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nature Neurosci. 3, 22–29 (2000).
Reid, T., Bathoorn, A., Ahmadian, M. R. & Collard, J. G. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J. Biol. Chem. 274, 33587–33593 (1999).
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Bishop, A. L. & Hall, A. Rho GTPases and their effector proteins . Biochem. J. 348, 241– 255 (2000).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 11, 2295– 2322 (1997).
Mammoto, A. et al. Interactions of drebrin and gephyrin with profilin. Biochem. Biophys. Res. Commun. 243, 86– 89 (1998).
Schluter, K., Jockusch, B. M. & Rothkegel, M. Profilins as regulators of actin dynamics. Biochim. Biophys. Acta 1359, 97–109 (1997).
Witke, W. et al. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO J. 17, 967–976 (1998).
Snyder, S. H. et al. Neural actions of immunophilin ligands. Trends Pharmacol. Sci. 19, 21–26 (1998).
Steward, O. mRNA localization in neurons: a multipurpose mechanism? Neuron 18, 9–12 (1997 ).
Racca, C., Gardiol, A. & Triller, A. Dendritic and postsynaptic localizations of glycine receptor α subunit mRNAs. J. Neurosci. 17, 1691–1700 (1997).
Kirsch, J. & Betz, H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature 392 , 717–720 (1998). These authors proposed the activation model for glycine synaptogenesis. The model that glycine release from nerve terminals causes postsynaptic depolarization, voltage-dependent calcium channel activation and gephyrin accumulation.
Levi, S., Vannier, C. & Triller, A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J. Cell Sci. 111, 335 –345 (1998).
Levi, S., Chesnoy-Marchais, D., Sieghart, W. & Triller, A. Synaptic control of glycine and GABAA receptors and gephyrin expression in cultured motoneurons. J. Neurosci. 19, 7434–7449 (1999). Using mixed cultures of motoneurons and spinal interneurons, the authors suggest that the identity of the presynaptic element determines the postsynaptic accumulation of glycine and GABA A receptors but not of gephyrin.
Taleb, O. & Betz, H. Expression of the human glycine receptor α1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 13, 1318 –1324 (1994).
Maammar, M., Rodeau, J. L. & Taleb, O. Permeation and gating of α1 glycine-gated channels expressed at low and high density in Xenopus oocyte. FEBS Lett. 414, 99–104 ( 1997).
Lim, R., Alvarez, F. J. & Walmsley, B. Quantal size is correlated with receptor cluster area at glycinergic synapses in the rat brainstem. J. Physiol. 516, 505–512 (1999).
Item, C. & Sieghart, W. Binding of γ-aminobutyric acidA receptors to tubulin. J. Neurochem. 63, 1119–1125 (1994).
Kannenberg, K., Baur, R. & Sigel, E. Proteins associated with α1 subunit-containing GABAA receptors from bovine brain. J. Neurochem. 68, 1352 –1360 (1997).
Triller, A., Cluzeaud, F. & Korn, H. Gamma-aminobutyric acid-containing terminals can be apposed to glycine receptors at central synapses. J. Cell Biol. 104, 947–956 ( 1987).
Cabot, J. B., Bushnell, A., Alessi, V. & Mendell, N. R. Postsynaptic gephyrin immunoreactivity exhibits a nearly one-to-one correspondence with γ-aminobutyric acid-like immunogold-labeled synaptic inputs to sympathetic preganglionic neurons. J. Comp. Neurol. 356, 418– 432 (1995).
Todd, A. J., Spike, R. C., Chong, D. & Neilson, M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. Eur. J. Neurosci. 7, 1–11 (1995).
Sassoe-Pognetto, M. et al. Co-localization of gephyrin and GABAA-receptor subunits in the rat retina. J. Comp. Neurol. 357, 1–14 (1995).
Sassoe-Pognetto, M. & Fritschy, J. M. Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses. Eur. J. Neurosci. 12, 2205–2210 (2000).
Essrich, C., Lorez, M., Benson, J. A., Fritschy, J. M. & Luscher, B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nature Neurosci. 1, 563–571 ( 1998).Mice that lack GABA A receptor γ2 subunits showed significant reductions of synaptic GABA A receptors clustering in cultured cortical neurons. Blocking the expression of gephyrin isoforms using antisense oligonucleotides had similar effects.
Craig, A. M., Banker, G., Chang, W., McGrath, M. E. & Serpinskaya, A. S. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J. Neurosci. 16, 3166–3177 (1996).
Kneussel, M. et al. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J. Neurosci. 19, 9289–9297 (1999).
Baer, K. et al. Postsynaptic clustering of γ-aminobutyric acid type A receptors by the γ3 subunit in vivo. Proc. Natl Acad. Sci. USA 96, 12860–12865 ( 1999).
Fischer, F. et al. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J. Comp. Neurol. 427, 634–648 ( 2000).
Kirsch, J. & Betz, H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton . J. Neurosci. 15, 4148– 4156 (1995).
Allison, D. W., Chervin, A. S., Gelfand, V. I. & Craig, A. M. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J. Neurosci. 20, 4545– 4554 (2000).
Meier, J., De Chaldee, M., Triller, A. & Vannier, C. Functional heterogeneity of gephyrins. Mol. Cell Neurosci. 16, 566–577 (2000). The authors provide evidence that some of the spliced variants of gephyrin have distinct functions. Specifically, not all of the spliced variants of gephyrin can bind the glycine receptor β-subunit. Furthermore, spliced variants can also have distinct subcellular localization in neurons.
Knuesel, I. et al. Short communication: altered synaptic clustering of GABA A receptors in mice lacking dystrophin (mdx mice). Eur. J. Neurosci. 11, 4457–4462 (1999).
Wang, H. B., Bedford, F. K., Brandon, N. J., Moss, S. J. & Olsen, R. W. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature 397, 69–72 ( 1999).
Wang, H. & Olsen, R. W. Binding of the GABAA receptor-associated protein (GABARAP) to microtubules and microfilaments suggests involvement of the cytoskeleton in GABARAP–GABAA receptor interaction. J. Neurochem. 75, 644– 655 (2000).
Mei, X., Sweatt, A. J. & Hammarback, J. A. Regulation of microtubule-associated protein 1B (MAP1B) subunit composition. J. Neurosci. Res. 62, 56–64 (2000).
Mann, S. S. & Hammarback, J. A. Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J. Biol. Chem. 269, 11492–11497 (1994).
Sagiv, Y., Legesse-Miller, A., Porat, A. & Elazar, Z. GATE-16, a membrane transport modulator, interacts with NSF and the Golgi v-SNARE GOS-28. EMBO J. 19, 1494– 1504 (2000).
Legesse-Miller, A., Sagiv, Y., Glozman, R. & Elazar, Z. Aut7p, a soluble autophagic factor, participates in multiple membrane trafficking processes. J. Biol. Chem. 275, 32966– 32973 (2000).
Kneussel, M. et al. The γ-aminobutyric acid type A receptor (GABAA R)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc. Natl Acad. Sci. USA 97, 8594–8599 ( 2000).The distribution and interaction of GABA A -receptor-associated protein (GABARAP) and gephyrin were compared in cultured neurons. GABARAP was found almost exclusively in intracellular compartments compared with synaptic sites where a much greater abundance of gephyrin was demonstrated. This distribution is consistent with a role for GABARAP in GABA A receptor transport rather than synaptic anchoring.
Chen, L., Wang, H., Vicini, S. & Olsen, R. W. The γ-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc. Natl Acad. Sci. USA 97, 11557– 11562 (2000).
Hanley, J. G., Koulen, P., Bedford, F., Gordon Weeks, P. R. & Moss, S. J. The protein MAP-1B links GABA C receptors to the cytoskeleton at retinal synapses. Nature 397, 66–69 ( 1999).This study showed a specific interaction between the GABA C receptor ρ1 and ρ2 subunits with the microtubule-associated protein MAP1B. Complexes of MAP1B and GABA C receptors were found in retinal bipolar neurons and facilitated the interaction of these receptors with the cytoskeleton.
Hammarback, J. A. in Brain Microtubule Associated Proteins: Modifications in Disease (eds Avila, J., Kosik, K. & Brandt, R.) 1–17 (Harwood, Amsterdam, 2001).
Billups, D., Hanley, J. G., Orme, M., Attwell, D. & Moss, S. J. GABAC receptor sensitivity is modulated by interaction with MAP1B. J. Neurosci. 20, 8643–8650 (2000).
Hanley, J. G., Jones, E. M. & Moss, S. J. GABA receptor rho1 subunit interacts with a novel splice variant of the glycine transporter, GLYT-1. J. Biol. Chem. 275, 840–846 ( 2000).
Connolly, C. N. et al. Cell surface stability of γ-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition . J. Biol. Chem. 274, 36565– 36572 (1999).
Chapell, R., Bueno, O. F., Alvarez-Hernandez, X., Robinson, L. C. & Leidenheimer, N. J. Activation of protein kinase C induces γ-aminobutyric acid type A receptor internalization in Xenopus oocytes. J. Biol. Chem. 273, 32595–32601 (1998).
Ghansah, E. & Weiss, D. S. Modulation of GABAA receptors by benzodiazepines and barbiturates is autonomous of PKC activation . Neuropharmacology 40, 327– 333 (2001).
Filippova, N., Sedelnikova, A., Zong, Y., Fortinberry, H. & Weiss, D. S. Regulation of recombinant γ-aminobutyric acid (GABAA and GABAC) receptors by protein kinase C . Mol. Pharmacol. 57, 847– 856 (2000).
Kittler, J. T. et al. Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using γ2 subunit green fluorescent protein chimeras. Mol. Cell. Neurosci. 16, 440–452 (2000).
Kittler, J. T. et al. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J. Neurosci. 20, 7972–7977 (2000). Interactions between β1–3 and γ2 GABA A receptor subunits and the adaptin AP2 complex were found in hippocampal neurons. Blocking this interaction caused a large time-dependent increase in the amplitude of miniature inhibitory postsynaptic current, suggesting that synaptic GABA A receptors undergo constitutive endocytosis.
Marsh, M. & McMahon, H. T. The structural era of endocytosis . Science 285, 215–220 (1999).
Meyer, T. & Shen, K. In and out of the postsynaptic region: signalling proteins on the move. Trends Cell Biol. 10, 238–244 (2000).
Wan, Q. et al. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature 388, 686– 690 (1997).The effect of insulin treatment of recombinant and native GABA A receptors was evaluated. Insulin promoted increased cell-surface expression of GABA A receptors in both HEK cells and in cultured hippocampal neurons. The amplitudes of miniature inhibitory postsynaptic currents were also increased by insulin without affecting their kinetics.
Moss, S. J. & Smart, T. G. Modulation of amino acid-gated ion channels by protein phosphorylation. Int. Rev. Neurobiol. 39, 1–52 (1996).
Smart, T. G. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr. Opin. Neurobiol. 7, 358–367 (1997).
McDonald, B. J. et al. Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nature Neurosci. 1, 23–28 (1998).
Moss, S. J., Doherty, C. A. & Huganir, R. L. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the β1, γ2S, and γ 2L subunits of the γ-aminobutyric acid type A receptor. J. Biol. Chem. 267, 14470–14476 (1992).
Moss, S. J., Smart, T. G., Blackstone, C. D. & Huganir, R. L. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science 257, 661– 665 (1992).
McDonald, B. J. & Moss, S. J. Differential phosphorylation of intracellular domains of γ-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J. Biol. Chem. 269, 18111– 18117 (1994).
McDonald, B. J. & Moss, S. J. Conserved phosphorylation of the intracellular domains of GABAA receptor β2 and β3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase . Neuropharmacology 36, 1377– 1385 (1997).
Brandon, N. J. et al. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J. Neurosci. 19, 9228–9234 ( 1999).Using affinity purification and gel-overlay assays, a specific association of PKC and RACK1 with β1–3 GABA A receptor subunits was observed. Both proteins bind independently but directly to the β-subunit intracellular domains. Immunoprecipitation showed that both PKC and RACK1 are intimately associated with native GABA A receptors in neurons.
Mochly-Rosen, D. & Gordon, A. S. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 12, 35–42 ( 1998).
Chang, B. Y., Conroy, K. B., Machleder, E. M. & Cartwright, C. A. RACK1, a receptor for activated C kinase and a homolog of the β subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol. Cell. Biol. 18, 3245– 3256 (1998).
Yarwood, S. J., Steele, M. R., Scotland, G., Houslay, M. D. & Bolger, G. B. The RACK1 signaling scaffold protein selectively interacts with the cAMP-specific phosphodiesterase PDE4D5 isoform . J. Biol. Chem. 274, 14909– 14917 (1999).
Brandon, N. J. et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J. Biol. Chem. 275, 38856–38862 (2000).
Smart, T. G., Thomas, P., Brandon, N. J. & Moss, S. J. in Pharmacology of GABA and Glycine Neurotransmission (ed. Mohler, H.) 195–225 (Springer–Verlag, Berlin, 2001).
Garner, C. C., Nash, J. & Huganir, R. L. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 10, 274–280 (2000).
Ye, B. et al. GRASP-1: a neuronal RasGEF associated with the AMPA receptor/GRIP complex. Neuron 26, 603– 617 (2000).
Kim, J. H., Liao, D., Lau, L. F. & Huganir, R. L. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron 20, 683–691 ( 1998).
Noel, J. et al. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 23, 365–376 (1999).
Nishimune, A. et al. NSF binding to GluR2 regulates synaptic transmission. Neuron 21, 87–97 ( 1998).
Husi, H., Ward, M. A., Choudhary, J. S., Blackstock, W. P. & Grant, S. G. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nature Neurosci. 3, 661–669 (2000).
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- BENZODIAZEPINES
-
Pharmacologically active molecules with sedative, anxiolytic and anticonvulsant effects. They act by binding to the GABA receptor and potentiate the response elicited by the transmitter.
- BARBITURATES
-
Pharmacologically active molecules with potent depressor effect in the central nervous system. They act by interacting with GABA receptors, potentiating the response elicited by the transmitter.
- INNER PLEXIFORM LAYER
-
Retinal layer formed by the synaptic contacts between the bipolar, the amacrine and the ganglion cells.
- YEAST TWO-HYBRID SCREENS
-
System used to determine the existence of direct interactions between proteins. It involves the use of plasmids that encode two hybrid proteins; one of them is fused to the GAL4 DNA-binding domain and the other one is fused to the GAL4 activation domain. The two proteins are expressed together in yeast and, if they interact, then the resulting complex will drive the expression of a reporter gene, commonly β-galactosidase.
- RAC/RHO GTPASES
-
Molecules related to the product of the oncogene ras, which are involved in controlling the polymerization and subsequent organization of actin.
- SH DOMAINS
-
Src-homology domains. They are involved in the interaction with phosphorylated tyrosine residues on other proteins (SH2 domains) or with proline-rich sections of other proteins (SH3 domains).
- CHANNEL-PERMEABILITY RATIO
-
A comparison of the ease with which two different permeant ions can pass through a channel.
- DYSTROPHIN
-
A protein that is absent in people with Duchenne muscular dystrophy. It is thought to participate in anchoring the cytoskeleton to the plasma membrane.
- ENDOSOME
-
Organelle that carries materials ingested by endocytosis and passes them to lysosomes for degradation or recycles them to the cell surface.
- CLATHRIN
-
One of the main protein components of the coats formed during membrane endocytosis.
- AP2 COMPLEX
-
Heterotetrameric complex composed of subunits called adaptins. It is one of the main components of the coats formed during membrane endocytosis.
- SMALL GTPases
-
Family of proteins capable of hydrolysing GTP, which include Rac, Rab, Ran, Rad, Rho and others. They subserve multiple cellular functions. For example, Rho and Rac are involved in the control of the cytoskeleton.
- DYNAMIN
-
A GTPase that is involved in endocytosis. It is thought to be involved in severing the connection between the nascent vesicle and the donor membrane.
- PDZ DOMAIN
-
A peptide-binding domain that is important for the organization of membrane proteins, particularly at cell–cell junctions, including synapses. They can bind to the carboxyl termini of proteins or can form dimers with other PDZ domains. PDZ domains are named after the proteins in which these sequence motifs were originally identified (PSD95, Discs-large, zona occludens-1).
- PSD95
-
A protein of the postsynaptic density, which can interact with NMDA receptors. It is thought to participate in regulating the spatial distribution of this receptor subtype.
- GRIP
-
(Glutamate-receptor-interacting protein). A protein that can interact with AMPA receptors. It is thought to participate in regulating the spatial distribution and targeting of this receptor subtype.
- RAS PROTEINS
-
A group of proteins involved in growth, differentiation and cellular signalling that require the binding of GTP to enter into their active state.
- GRASP
-
(GRIP1-associated scaffold protein). A guanine-nucleotide exchange factor that owing to its interaction with GRIP might link AMPA receptors to Ras signalling.
- SYNGAP
-
A synaptic Ras-GTPase activating protein that interacts with PSD95. It might participate in controlling the spatial distribution of NMDA receptors, as well as in regulating Ras signalling.
Rights and permissions
About this article
Cite this article
Moss, S., Smart, T. Constructing inhibitory synapses. Nat Rev Neurosci 2, 240–250 (2001). https://doi.org/10.1038/35067500
Issue Date:
DOI: https://doi.org/10.1038/35067500
This article is cited by
-
Nonlinear mechanism for the enhanced bursting activities induced by fast inhibitory autapse and reduced activities by fast excitatory autapse
Cognitive Neurodynamics (2023)
-
Roles of neuroligins in central nervous system development: focus on glial neuroligins and neuron neuroligins
Journal of Translational Medicine (2022)
-
Glycine encephalopathy
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2022)
-
Brain volume increase and neuronal plasticity underly predator-induced morphological defense expression in Daphnia longicephala
Scientific Reports (2021)
-
Assembly and maintenance of GABAergic and Glycinergic circuits in the mammalian nervous system
Neural Development (2018)