Key Points
-
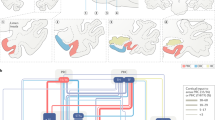
Comprehensive knowledge of the architecture of neuronal networks lies at the basis of understanding their functions. Although the anatomical connections between and within the hippocampal formation (HF) and the parahippocampal region (PHR) have been and still are being investigated extensively, for several reasons some of the PHR–HF network connections have become underexposed and this probably results in biased functional concepts.
-
We present a comprehensive interactive knowledge base of all anatomically established PHR–HF connections in the rat. Using this knowledge base, the PHR–HF circuitry is discussed and special attention is paid to underexposed connections.
-
The role of some of these underexposed connections is discussed in relation to three topics that are strongly associated with the PHR–HF network: memory formation, navigation and temporal dynamics.
-
Generally it is thought that only the HF is involved in memory formation, through associating different types of information. Based on the connections observed in the interactive diagram of the knowledge base, we pose that the entorhinal cortex associates information prehippocampally at a more generic level than the HF.
-
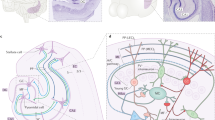
The CA3 recurrent network is the most prominent auto-associative network in the hippocampus and is implicated in pattern-separation and pattern-completion tasks that are relevant for memory. However, there are also recurrent networks in the hilus of the dentate gyrus, CA1 and the subiculum. They might also serve a unique role in memory, but they receive little attention.
-
Detailed anatomical knowledge lies at the basis of the discovery of grid cells that are important for navigation. Based on details of the entorhinal–hippocampal connections, we propose that there will be differences in the modulation of firing patterns along the transverse axis in CA1 and the subiculum. The proximal part of CA1 and the distal subiculum will preferentially process spatial information, whereas the distal part of CA1 and the proximal subiculum will process non-spatial information.
-
Although most track-tracing studies do not reveal whether a connection is excitatory or inhibitory, we suggest that some of the known connections between regions of the network are likely to be identified as inhibitory interneuron projections, based on their layer of origin. These long-range GABA (γ-aminobutyric acid)-ergic connections probably mediate interregional binding.
-
Sources of detailed knowledge, such as that presented in this Review and the accompanying interactive diagram, will prevent the loss of valuable knowledge and hopefully inspire creative minds to come up with new solutions for outstanding problems in the field.
Abstract
Converging evidence suggests that each parahippocampal and hippocampal subregion contributes uniquely to the encoding, consolidation and retrieval of declarative memories, but their precise roles remain elusive. Current functional thinking does not fully incorporate the intricately connected networks that link these subregions, owing to their organizational complexity; however, such detailed anatomical knowledge is of pivotal importance for comprehending the unique functional contribution of each subregion. We have therefore developed an interactive diagram with the aim to display all of the currently known anatomical connections of the rat parahippocampal–hippocampal network. In this Review, we integrate the existing anatomical knowledge into a concise description of this network and discuss the functional implications of some relatively underexposed connections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ramón y Cajal, S. Estructura del asta de Ammon y fascia dentata. Anales de la Sociedad Española de Historia Natural 22, 53–126 (1893).
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957).
Zola-Morgan, S., Squire, L. R., Amaral, D. G. & Suzuki, W. A. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J. Neurosci. 9, 4355–4370 (1989).
Crick, F. & Koch, C. A framework for consciousness. Nature Neurosci. 6, 119–126 (2003).
Amaral, D. G. & Lavenex, P. in The Hippocampus Book (eds Andersen, P., Morris, R., Amaral, D. G., Bliss, T. & O'Keefe, J.) 37–114 (Oxford Univ. Press, New York, 2007).
Witter, M. P. & Amaral, D. G. in The Rat Nervous System 3rd edn (ed. Paxinos, G.) 637–703 (Elsevier Academic Press, San Diego, 2004). This book chapter gives an extensive description of cells and projections in the HF and the PHR.
Burwell, R. D. & Witter, M. P. in The Parahippocampal Region: Organization and Role in Cognitive Function (eds Witter, M. P. & Wouterlood, F. G.) 35–60 (Oxford Univ. Press, New York, 2002).
Bird, C. M. & Burgess, N. The hippocampus and memory: insights from spatial processing. Nature Rev. Neurosci. 9, 182–194 (2008). This paper provides a comprehensive summary of arguments that support the thesis that studies on the role of the rodent hippocampus in navigation bear on our understanding of memory.
Brown, M. W. & Aggleton, J. P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Rev. Neurosci. 2, 51–61 (2001).
Bussey, T. J. & Saksida, L. M. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus 17, 898–908 (2007). This paper provides a critical but stimulating perspective on the idea that the medial temporal lobe system is involved only in memory, with special emphasis on the functions of the perirhinal cortex.
Eichenbaum, H. A cortical-hippocampal system for declarative memory. Nature Rev. Neurosci. 1, 41–50 (2000).
Eichenbaum, H., Yonelinas, A. P. & Ranganath, C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152 (2007). This paper reviews current concepts of how familiarity and recollection memory are supported by regions in the medial temporal lobe and proposes that there is a distinction between recollection and familiarity in specific regions of the medial temporal lobe.
Hasselmo, M. E., Fransen, E., Dickson, C. & Alonso, A. A. Computational modeling of entorhinal cortex. Ann. NY Acad. Sci. 911, 418–446 (2000).
Martin, S. J. & Clark, R. E. The rodent hippocampus and spatial memory: from synapses to systems. Cell. Mol. Life Sci. 64, 401–431 (2007).
Squire, L. R., Stark, C. E. & Clark, R. E. The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004).
Burwell, R. D. & Amaral, D. G. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J. Comp. Neurol. 398, 179–205 (1998).
Burwell, R. D. & Amaral, D. G. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J. Comp. Neurol. 391, 293–321 (1998).
Naber, P. A., Caballero-Bleda, M., Jorritsma-Byham, B. & Witter, M. P. Parallel input to the hippocampal memory system through peri- and postrhinal cortices. Neuroreport 8, 2617–2621 (1997).
Swanson, L. W. & Kohler, C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J. Neurosci. 6, 3010–3023 (1986).
Kohler, C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J. Comp. Neurol. 246, 149–169 (1986).
Deacon, T. W., Eichenbaum, H., Rosenberg, P. & Eckmann, K. W. Afferent connections of the perirhinal cortex in the rat. J. Comp. Neurol. 220, 168–190 (1983).
Kerr, K. M., Agster, K. L., Furtak, S. C. & Burwell, R. D. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus 17, 697–708 (2007).
Furtak, S. C., Wei, S. M., Agster, K. L. & Burwell, R. D. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus 17, 709–722 (2007).
van Groen, T. & Wyss, J. M. The connections of presubiculum and parasubiculum in the rat. Brain Res. 518, 227–243 (1990).
Wyss, J. M. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J. Comp. Neurol. 199, 495–512 (1981).
Lingenhohl, K. & Finch, D. M. Morphological characterization of rat entorhinal neurons in vivo: soma-dendritic structure and axonal domains. Exp. Brain Res. 84, 57–74 (1991).
Honda, Y. & Ishizuka, N. Organization of connectivity of the rat presubiculum: I. Efferent projections to the medial entorhinal cortex. J. Comp. Neurol. 473, 463–484 (2004).
Van Haeften, T., Wouterlood, F. G., Jorritsma-Byham, B. & Witter, M. P. GABAergic presubicular projections to the medial entorhinal cortex of the rat. J. Neurosci. 17, 862–874 (1997).
Kohler, C. Intrinsic connections of the retrohippocampal region in the rat brain: III. The lateral entorhinal area. J. Comp. Neurol. 271, 208–228 (1988).
Segal, M. Afferents to the entorhinal cortex of the rat studied by the method of retrograde transport of horseradish peroxidase. Exp. Neurol. 57, 750–765 (1977).
van Groen, T. & Wyss, J. M. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Res. 529, 165–177 (1990).
Kohler, C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J. Comp. Neurol. 236, 504–522 (1985).
Caballero-Bleda, M. & Witter, M. P. Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: an anterograde tracing study in the rat. J. Comp. Neurol. 328, 115–129 (1993).
Honda, Y., Umitsu, Y. & Ishizuka, N. Organization of connectivity of the rat presubiculum: II. Associational and commissural connections. J. Comp. Neurol. 506, 640–658 (2008).
Van Haeften, T., Wouterlood, F. G. & Witter, M. P. Presubicular input to the dendrites of layer-V entorhinal neurons in the rat. Ann. NY Acad. Sci. 911, 471–473 (2000).
Kohler, C., Shipley, M. T., Srebro, B. & Harkmark, W. Some retrohippocampal afferents to the entorhinal cortex. Cells of origin as studied by the HRP method in the rat and mouse. Neurosci. Lett. 10, 115–120 (1978).
Dolorfo, C. L. & Amaral, D. G. Entorhinal cortex of the rat: organization of intrinsic connections. J. Comp. Neurol. 398, 49–82 (1998).
Baks-Te-Bulte, L., Wouterlood, F. G., Vinkenoog, M. & Witter, M. P. Entorhinal projections terminate onto principal neurons and interneurons in the subiculum: a quantitative electron microscopical analysis in the rat. Neuroscience 136, 729–739 (2005).
Deller, T., Martinez, A., Nitsch, R. & Frotscher, M. A novel entorhinal projection to the rat dentate gyrus: direct innervation of proximal dendrites and cell bodies of granule cells and GABAergic neurons. J. Neurosci. 16, 3322–3333 (1996). This paper described an underexposed projection from the deep layers of the EC to the DG, which should fundamentally alter our concept of the EC (see also reference 96).
Dolorfo, C. L. & Amaral, D. G. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J. Comp. Neurol. 398, 25–48 (1998).
Hjorth-Simonsen, A. Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. J. Comp. Neurol. 146, 219–232 (1972).
Kajiwara, R. et al. Convergence of entorhinal and CA3 inputs onto pyramidal neurons and interneurons in hippocampal area CA1 - An anatomical study in the rat. Hippocampus 18, 266–280 (2007).
Kohler, C. A projection from the deep layers of the entorhinal area to the hippocampal formation in the rat brain. Neurosci. Lett. 56, 13–19 (1985).
Nafstad, P. H. An electron microscope study on the termination of the perforant path fibres in the hippocampus and the fascia dentata. Z. Zellforsch. Mikrosk. Anat. 76, 532–542 (1967).
Ruth, R. E., Collier, T. J. & Routtenberg, A. Topography between the entorhinal cortex and the dentate septotemporal axis in rats: I. Medial and intermediate entorhinal projecting cells. J. Comp. Neurol. 209, 69–78 (1982).
Ruth, R. E., Collier, T. J. & Routtenberg, A. Topographical relationship between the entorhinal cortex and the septotemporal axis of the dentate gyrus in rats: II. Cells projecting from lateral entorhinal subdivisions. J. Comp. Neurol. 270, 506–516 (1988).
Segal, M. & Landis, S. Afferents to the hippocampus of the rat studied with the method of retrograde transport of horseradish peroxidase. Brain Res. 78, 1–15 (1974).
Steward, O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J. Comp. Neurol. 167, 285–314 (1976).
Tamamaki, N. & Nojyo, Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J. Comp. Neurol. 353, 379–390 (1995).
Tamamaki, N. Organization of the entorhinal projection to the rat dentate gyrus revealed by Dil anterograde labeling. Exp. Brain Res. 116, 250–258 (1997).
Witter, M. P., Griffioen, A. W., Jorritsma-Byham, B. & Krijnen, J. L. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci. Lett. 85, 193–198 (1988).
Naber, P. A., Lopes da Silva, F. H. & Witter, M. P. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus 11, 99–104 (2001).
Sporns, O. & Tononi, G. in Handbook of Brain Connectivity (eds Jirsa, V. K. & McIntosh, A. R.) 117–147 (Springer, Berlin, 2007). In this book chapter the authors clearly explain segregation and integration and describe a method for quantifying structural connection patterns as global measures of brain dynamics.
Tamamaki, N. & Nojyo, Y. Projection of the entorhinal layer II neurons in the rat as revealed by intracellular pressure-injection of neurobiotin. Hippocampus 3, 471–480 (1993).
Braak, H. On the structure of the human archicortex. I. The cornu ammonis. A Golgi and pigmentarchitectonic study. Cell Tissue Res. 152, 349–383 (1974).
Honda, Y., Umitsu, Y. & Ishizuka, N. Topographic projections of perforant path from entorhinal area to CA1 and subiculum in the rat. Neurosci. Res. 24 (Suppl.), S101 (2000).
Naber, P. A., Witter, M. P. & Lopes da Silva, F. H. Evidence for a direct projection from the postrhinal cortex to the subiculum in the rat. Hippocampus 11, 105–117 (2001).
Witter, M. P. & Groenewegen, H. J. Laminar origin and septotemporal distribution of entorhinal and perirhinal projections to the hippocampus in the cat. J. Comp. Neurol. 224, 371–385 (1984).
Witter, M. P. A survey of the anatomy of the hippocampal formation, with emphasis on the septotemporal organization of its intrinsic and extrinsic connections. Adv. Exp. Med. Biol. 203, 67–82 (1986).
Witter, M. P., Holtrop, R. & van de Loosdrecht, A. A. Direct projections from the periallocortical subicular complex to the fascia dentata in the rat. Neurosci. Res. Commun. 2, 61–68 (1988).
Beckstead, R. M. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res. 152, 249–264 (1978).
Kosel, K. C., Van Hoesen, G. W. & Rosene, D. L. A direct projection from the perirhinal cortex (area 35) to the subiculum in the rat. Brain Res. 269, 347–351 (1983).
Ishizuka, N., Weber, J. & Amaral, D. G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J. Comp. Neurol. 295, 580–623 (1990).
Laurberg, S. Commissural and intrinsic connections of the rat hippocampus. J. Comp. Neurol. 184, 685–708 (1979).
Laurberg, S. & Sorensen, K. E. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentatae and subfield CA3). Brain Res. 212, 287–300 (1981).
Amaral, D. G., Dolorfo, C. & varez-Royo, P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus 1, 415–435 (1991).
Swanson, L. W., Sawchenko, P. E. & Cowan, W. M. Evidence for collateral projections by neurons in Ammon's horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. J. Neurosci. 1, 548–559 (1981).
Tamamaki, N. & Nojyo, Y. Disposition of the slab-like modules formed by axon branches originating from single CA1 pyramidal neurons in the rat hippocampus. J. Comp. Neurol. 291, 509–519 (1990).
van Groen, T. & Wyss, J. M. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J. Comp. Neurol. 302, 515–528 (1990).
Buckmaster, P. S., Strowbridge, B. W. & Schwartzkroin, P. A. A comparison of rat hippocampal mossy cells and CA3c pyramidal cells. J. Neurophysiol. 70, 1281–1299 (1993).
Li, X. G., Somogyi, P., Ylinen, A. & Buzsaki, G. The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol. 339, 181–208 (1994).
Swanson, L. W., Wyss, J. M. & Cowan, W. M. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J. Comp. Neurol. 181, 681–715 (1978).
Wittner, L., Henze, D. A., Zaborszky, L. & Buzsaki, G. Hippocampal CA3 pyramidal cells selectively innervate aspiny interneurons. Eur. J. Neurosci. 24, 1286–1298 (2006).
Wittner, L., Henze, D. A., Zaborszky, L. & Buzsaki, G. Three-dimensional reconstruction of the axon arbor of a CA3 pyramidal cell recorded and filled in vivo. Brain Struct. Funct. 212, 75–83 (2007).
Cenquizca, L. A. & Swanson, L. W. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev. 56, 1–26 (2007).
Finch, D. M., Nowlin, N. L. & Babb, T. L. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res. 271, 201–216 (1983).
Sik, A., Tamamaki, N. & Freund, T. F. Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. Eur. J. Neurosci. 5, 1719–1728 (1993).
Siddiqui, A. H. & Joseph, S. A. CA3 axonal sprouting in kainate-induced chronic epilepsy. Brain Res. 1066, 129–146 (2005).
Hjorth-Simonsen, A. Some intrinsic connections of the hippocampus in the rat: an experimental analysis. J. Comp. Neurol. 147, 145–161 (1973).
Van, G. T. & Wyss, J. M. Species differences in hippocampal commissural connections: studies in rat, guinea pig, rabbit, and cat. J. Comp. Neurol. 267, 322–334 (1988).
Lorente de Nó, R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J. Psychol. Neurol. 46, 113–177 (1934).
Claiborne, B. J., Amaral, D. G. & Cowan, W. M. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 246, 435–458 (1986).
Frotscher, M., Seress, L., Schwerdtfeger, W. K. & Buhl, E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J. Comp. Neurol. 312, 145–163 (1991).
Deller, T., Nitsch, R. & Frotscher, M. Heterogeneity of the commissural projection to the rat dentate gyrus: a Phaseolus vulgaris leucoagglutinin tracing study. Neuroscience 75, 111–121 (1996).
Swanson, L. W. & Cowan, W. M. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J. Comp. Neurol. 172, 49–84 (1977).
Finch, D. M. & Babb, T. L. Demonstration of caudally directed hippocampal efferents in the rat by intracellular injection of horseradish peroxidase. Brain Res. 214, 405–410 (1981).
Harris, E., Witter, M. P., Weinstein, G. & Stewart, M. Intrinsic connectivity of the rat subiculum: I. Dendritic morphology and patterns of axonal arborization by pyramidal neurons. J. Comp. Neurol. 435, 490–505 (2001).
Witter, M. P., Ostendorf, R. H. & Groenewegen, H. J. Heterogeneity in the dorsal subiculum of the rat. Distinct neuronal zones project to different cortical and subcortical targets. Eur. J. Neurosci. 2, 718–725 (1990).
Kloosterman, F., Witter, M. P. & Van Haeften, T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J. Comp. Neurol. 455, 156–171 (2003).
Van Haeften, T., Jorritsma-Byham, B. & Witter, M. P. Quantitative morphological analysis of subicular terminals in the rat entorhinal cortex. Hippocampus 5, 452–459 (1995).
Naber, P. A. & Witter, M. P. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J. Comp. Neurol. 393, 284–297 (1998).
Honda, Y., Umitsu, Y. & Ishizuka, N. Efferent projections of the subiculum to the retrohippocampal and retrosplenial cortices of the rat. Neurosci. Res. 22 (Suppl.), S265 (1999).
Mayeaux, D. J. & Johnston, R. E. Discrimination of social odors and their locations: role of lateral entorhinal area. Physiol. Behav. 82, 653–662 (2004).
Van Haeften, T., Baks- Te-Bulte, L., Goede, P. H., Wouterlood, F. G. & Witter, M. P. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus 13, 943–952 (2003).
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006). This study provided evidence that information on location, direction and distance is integrated and updated in the grid cell network during navigation.
Koganezawa, N. et al. Significance of the deep layers of entorhinal cortex for transfer of both perirhinal and amygdala inputs to the hippocampus. Neurosci. Res. 61, 172–181 (2008).
Viskontas, I. V., Carr, V. A., Engel, S. A. & Knowlton, B. J. The neural correlates of recollection: hippocampal activation declines as episodic memory fades. Hippocampus 19, 265–272 (2008).
Rolls, E. T. An attractor network in the hippocampus: theory and neurophysiology. Learn. Mem. 14, 714–731 (2007). This paper provided a clear definition of the characteristic features of attractor networks and summarized data in support of the view that the hippocampus comprises an attractor network relevant for fast and efficient encoding and retrieval of trial-unique memories.
Nakazawa, K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218 (2002).
Gold, A. E. & Kesner, R. P. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus 15, 808–814 (2005).
McNaughton, B. L. et al. Distinct characteristics of CA1 place cells correlated with medial or lateral entorhinal cortex layer III input. Society for Neuroscience Abstract 391.3 (2008).
O'Keefe, J. Place units in the hippocampus of the freely moving rat. Exp. Neurol. 51, 78–109 (1976).
Kjelstrup, K. B. et al. Finite scale of spatial representation in the hippocampus. Science 321, 140–143 (2008).
Sharp, P. E. & Green, C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J. Neurosci. 14, 2339–2356 (1994).
Sharp, P. E. Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav. Brain Res. 85, 71–92 (1997).
Sharp, P. E. Subicular place cells expand or contract their spatial firing pattern to fit the size of the environment in an open field but not in the presence of barriers: comparison with hippocampal place cells. Behav. Neurosci. 113, 643–662 (1999).
Sharp, P. E. Multiple spatial/behavioral correlates for cells in the rat postsubiculum: multiple regression analysis and comparison to other hippocampal areas. Cereb. Cortex 6, 238–259 (1996).
O'Keefe, J. in The Hippocampus Book (eds Andersen, P., Morris, R., Amaral, D. G., Bliss, T. & O'Keefe, J.) 475–579 (Oxford Univ. Press, New York, 2007).
Taube, J. S. Place cells recorded in the parasubiculum of freely moving rats. Hippocampus 5, 569–583 (1995).
Jung, M. W., Wiener, S. I. & McNaughton, B. L. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 14, 7347–7356 (1994).
Maurer, A. P., Vanrhoads, S. R., Sutherland, G. R., Lipa, P. & McNaughton, B. L. Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus 15, 841–852 (2005).
Touretzky, D. S. & Redish, A. D. Theory of rodent navigation based on interacting representations of space. Hippocampus 6, 247–270 (1996).
Sharp, P. E. Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus 9, 432–443 (1999).
Remondes, M. & Schuman, E. M. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431, 699–703 (2004).
Brun, V. H. et al. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron 57, 290–302 (2008).
Quirk, G. J., Muller, R. U., Kubie, J. L. & Ranck, J. B. Jr. The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J. Neurosci. 12, 1945–1963 (1992).
Frank, L. M., Brown, E. N. & Wilson, M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27, 169–178 (2000).
Fyhn, M., Molden, S., Witter, M. P., Moser, E. I. & Moser, M. B. Spatial representation in the entorhinal cortex. Science 305, 1258–1264 (2004).
Brun, V. H. et al. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18, 1200–1212 (2008).
Taube, J. S., Muller, R. U. & Ranck, J. B. Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci. 10, 436–447 (1990).
Mizumori, S. J. & Williams, J. D. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J. Neurosci. 13, 4015–4028 (1993).
Taube, J. S. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci. 15, 70–86 (1995).
Stackman, R. W. & Taube, J. S. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J. Neurosci. 17, 4349–4358 (1997).
Sharp, P. E. & Koester, K. Lesions of the mammillary body region severely disrupt the cortical head direction, but not place cell signal. Hippocampus 18, 766–784 (2008). This study provides evidence that the mammillary bodies provide important information for the head-direction cells but not for the place cells in the hippocampus.
Chen, L. L., Lin, L. H., Green, E. J., Barnes, C. A. & McNaughton, B. L. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp. Brain Res. 101, 8–23 (1994).
Wiener, S. I. Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J. Neurosci. 13, 3802–3817 (1993).
Calton, J. L. et al. Hippocampal place cell instability after lesions of the head direction cell network. J. Neurosci. 23, 9719–9731 (2003).
McNaughton, B. L., Barnes, C. A., Meltzer, J. & Sutherland, R. J. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res. 76, 485–496 (1989).
Brun, V. H. et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science 296, 2243–2246 (2002).
Witter, M. P. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav. Brain Res. 174, 251–264 (2006).
Buzsaki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Levy, W. B. & Steward, O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 175, 233–245 (1979).
Wilson, M. A. & McNaughton, B. L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994).
Mann, E. O. & Paulsen, O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 30, 343–349 (2007). This paper reviews the mechanism by which GABAergic interneurons control the spike timing of other neurons and synchronize network activity.
Cobb, S. R., Buhl, E. H., Halasy, K., Paulsen, O. & Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78 (1995).
Klausberger, T. et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848 (2003).
Klausberger, T. et al. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nature Neurosci. 7, 41–47 (2004).
Tukker, J. J., Fuentealba, P., Hartwich, K., Somogyi, P. & Klausberger, T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J. Neurosci. 27, 8184–8189 (2007).
Jinno, S. et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 27, 8790–8804 (2007). This paper provides evidence that particular long-range GABAergic projection neurons are important for coordinating oscillation across brain areas.
Gulyas, A. I., Hajos, N., Katona, I. & Freund, T. F. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur. J. Neurosci. 17, 1861–1872 (2003).
Losonczy, A., Zhang, L., Shigemoto, R., Somogyi, P. & Nusser, Z. Cell type dependence and variability in the short-term plasticity of EPSCs in identified mouse hippocampal interneurones. J. Physiol. 542, 193–210 (2002).
Sik, A., Penttonen, M., Ylinen, A. & Buzsaki, G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J. Neurosci. 15, 6651–6665 (1995).
Vida, I., Halasy, K., Szinyei, C., Somogyi, P. & Buhl, E. H. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J. Physiol. 506, 755–773 (1998).
Tulving, E. in Organization of Memory (eds Tulving, E. & Donaldson, W.) 381–403 (Academic, New York, 1972).
Goulet, S. & Murray, E. A. Neural substrates of crossmodal association memory in monkeys: the amygdala versus the anterior rhinal cortex. Behav. Neurosci. 115, 271–284 (2001).
Mayes, A., Montaldi, D. & Migo, E. Associative memory and the medial temporal lobes. Trends Cogn. Sci. 11, 126–135 (2007).
Manns, J. R., Howard, M. W. & Eichenbaum, H. Gradual changes in hippocampal activity support remembering the order of events. Neuron 56, 530–540 (2007).
Fortin, N. J., Agster, K. L. & Eichenbaum, H. B. Critical role of the hippocampus in memory for sequences of events. Nature Neurosci. 5, 458–462 (2002).
Kesner, R. P., Gilbert, P. E. & Barua, L. A. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav. Neurosci. 116, 286–290 (2002).
Lisman, J. E. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 22, 233–242 (1999).
Bussey, T. J., Saksida, L. M. & Murray, E. A. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Q. J. Exp. Psychol. B 58, 269–282 (2005).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
McCormick, D. A. & Contreras, D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 63, 815–846 (2001).
Honea, R., Crow, T. J., Passingham, D. & Mackay, C. E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 162, 2233–2245 (2005).
Campbell, S. & MacQueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 29, 417–426 (2004).
Zaborszky, L., Wouterlood, F. G. & Lanciego, J. L. Neuroanatomical Tract-Tracing: Molecules, Neurons, and Systems (Springer, 2006). This book provides excellent guidance on tract-tracing methods.
Wickersham, I. R., Finke, S., Conzelmann, K. K. & Callaway, E. M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nature Methods 4, 47–49 (2007).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits. Neuron 57, 634–660 (2008). This paper showed that a genetic approach can be an additional tool, next to anatomical and physiological methods, for constructing circuit diagrams.
Acknowledgements
The authors would like to thank E. Moser and L. Colgin for providing helpful comments on an earlier version of this article. N.L.M.C. acknowledges a personal grant from the Netherlands Organization for scientific research (NWO) — grant number 903-47-074.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Temporal dynamics
-
Properties of neurons in a network, such as precise spike times and firing rates, that facilitate information transfer.
- Convergence
-
When inputs from different brain regions congregate on to single cells or on to a local network in another region.
- Reciprocal connections
-
Bidirectional, equivalent connections between two areas, networks or neurons.
- Perforant pathway
-
Axons that originate in the superficial layers of the EC and are distributed to all fields of the hippocampus.
- Divergence
-
When one brain region sends projections to several different brain regions.
- Mossy fibres
-
The main projection of DG granular cells to CA3; characterized by high concentrations of zinc.
- Schaffer collaterals
-
The axon collaterals of the CA3 pyramidal cells that project to CA1.
- Auto-associative network
-
A network of neurons with axon collaterals that terminate on dendrites of the parent cell.
- Place cells
-
Principal neurons in the hippocampus and parahippocampus that fire whenever an animal is in a specific location in an environment (corresponding to the cell's 'place field').
- Grid cells
-
Neurons in the entorhinal cortex that fire strongly when an animal is at one of several specific locations in an environment and that are organized in a grid-like fashion.
- Head-direction cells
-
Neurons that fire only when the animal's head points in a specific direction in an environment.
- Mammillary bodies
-
A pair of nuclei in the hypothalamus, strongly connected to the HF and the anterior complex of the thalamus, that are involved in recognition memory.
- Theta oscillations
-
Rhythmical changes at 5–12 Hz in network activity, as observed in the electroencephalogram, characteristic of the hippocampal network communicating with various cortical and subcortical networks in the brain.
- Gamma oscillations
-
Rhythmical oscillations of 25–70 Hz observed in the electroencephalogram.
- Ripple oscillations
-
Short-lasting bursts of field oscillations (∼140–200 Hz) in the mammalian hippocampus and parahippocampus that occur during rest or slow-wave sleep.
Rights and permissions
About this article
Cite this article
van Strien, N., Cappaert, N. & Witter, M. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci 10, 272–282 (2009). https://doi.org/10.1038/nrn2614
Issue Date:
DOI: https://doi.org/10.1038/nrn2614
This article is cited by
-
Hippocampus-centred grey matter covariance networks predict the development and reversion of mild cognitive impairment
Alzheimer's Research & Therapy (2023)
-
Immediate visual reproduction negatively correlates with brain entropy of parahippocampal gyrus and inferior occipital gyrus in bipolar II disorder adolescents
BMC Psychiatry (2023)
-
Differentiation between fetal and postnatal iron deficiency in altering brain substrates of cognitive control in pre-adolescence
BMC Medicine (2023)
-
Extracellular vesicles from mesenchymal stem cells reduce neuroinflammation in hippocampus and restore cognitive function in hyperammonemic rats
Journal of Neuroinflammation (2023)
-
Novel effects of Ras-MAPK pathogenic variants on the developing human brain and their link to gene expression and inhibition abilities
Translational Psychiatry (2023)