Key Points
-
Endogenous antimicrobial peptide molecules of less than 100 amino acids are an important component of innate immunity. The type and number of antimicrobial peptides varies from species to species. In humans and other primates, defensins (cationic antimicrobial peptides that are rich in cysteine residues) contribute to host defence against bacterial, fungal and viral infections.
-
Three families of defensins — α, β and θ — are found in primates. The α-defensins, which were first discovered in the 1960s but not sequenced until almost 20 years later, are most prominent in neutrophils and Paneth cells in the small intestine. β-defensins were initially identified in cattle. Later (in 1995) they were found in humans, and reported to protect the skin and the mucous membranes of the respiratory, genitourinary and gastrointestinal tracts. α- and β-defensins differ from each other in the spacing and pairing of their six conserved cysteines. θ-defensins were first identified in 1999 and are expressed only in PMNs and bone marrow in Old World monkeys, lesser apes and orangutans.
-
Unsurprisingly, some bacteria that infect humans can resist the antimicrobial activities of defensins. In Salmonella enterica serovar Typhimurium, the PhoP−PhoQ regulon controls the transcription of many genes involved in resistance to defensins and other antimicrobial molecules. Several factors that contribute to defensin resistance have been identified in Staphylococcus aureus, including MprF, a lysylphosphatidylglycerol synthase, which functions by decreasing the negative charge of the bacterial membrane, thereby decreasing binding of cationic defensins. Neisseria gonorrhoeae are naturally resistant to α-defensins.
-
The antimicrobial activity of defensins is mainly due to permeabilization of the target cell membrane. However, defensins also have a range of other important activities, including involvement in chemotaxis of monocytes, T cells and dendritic cells; the release of histamine from mast cells; opsonization; interaction with complement; and wound repair.
-
The antiviral properties of defensins are beginning to be studied more, and this research has led to the discovery that many α-defensins have lectin-like properties. The θ-defensins, the latest defensin family to be discovered, also have lectin-like properties and show broad-spectrum antiviral efficacy. The genes encoding these molecules (DEFT) are mutated α-defensin genes that arose in an Old World monkey. Humans, chimpanzees and gorillas do not express θ-defensin peptides because their DEFT genes acquired a mutation that introduced a premature stop codon into the signal sequence. A synthetic θ-defensin, retrocyclin 2, has activity against HIV-1 and HSV-2 and acts by inhibiting viral entry. The role of endogenous human α-defensins in resistance to HIV/AIDS is not yet clear.
Abstract
Defensins are endogenous, cysteine-rich antimicrobial peptides that contribute to host defence against bacterial, fungal and viral infections. There are three subfamilies of defensins in primates: α-defensins are most common in neutrophils and Paneth cells of the small intestine; β-defensins protect the skin and the mucous membranes of the respiratory, genitourinary and gastrointestinal tracts; and θ-defensins, which are expressed only in Old World monkeys, lesser apes and orangutans, are lectins with broad-spectrum antiviral efficacy. Here, their discovery and recent advances in understanding their properties and functions are described.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ganz, T. et al. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest 76, 1427–1435 (1985).
Lehrer, R. I. & Ganz, T. Defensins of vertebrate animals. Curr. Opin. Immunol. 14, 96–102 (2002).
Zanetti, M., Gennaro, R. & Romeo, D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374, 1–5 (1995).
Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75, 39–48 (2004).
Rice, W. G. et al. Defensin-rich dense granules of human neutrophils. Blood 70, 757–765 (1987).
Sorensen, O. E. et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 (2001).
Sorensen, O., Arnljots, K., Cowland, J. B., Bainton, D. F. & Borregaard, N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90, 2796–2803 (1997).
Bainton, D. F. Distinct granule populations in human neutrophils and lysosomal organelles identified by immuno-electron microscopy. J. Immunol. Methods 232, 153–168 (1999).
Borregaard, N. et al. Human neutrophil granules and secretory vesicles. Eur. J. Haematol. 51, 187–198 (1993).
Selsted, M. E. et al. Purification, primary structures, and antibacterial activities of β-defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 268, 6641–6648 (1993).
Zarember, K. A. et al. Host defense functions of proteolytically processed and parent (unprocessed) cathelicidins of rabbit granulocytes. Infect. Immun. 70, 569–576 (2002).
Selsted, M. E., Brown, D. M., DeLange, R. J., Harwig, S. S. & Lehrer, R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J. Biol. Chem. 260, 4579–4584 (1985).
Huttner, K. M., Lambeth, M. R., Burkin, H. R., Burkin, D. J. & Broad, T. E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene 206, 85–91 (1998).
Bals, R. & Wilson, J. M. Cathelicidins—a family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. 60, 711–720 (2003).
Termen, S. et al. Phylogeny, processing and expression of the rat cathelicidin rCRAMP: a model for innate antimicrobial peptides. Cell. Mol. Life Sci. 60, 536–549 (2003).
Gallo, R. L. et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272, 13088–13093 (1997).
Eisenhauer, P., Harwig, S. S., Szklarek, D., Ganz, T. & Lehrer, R. I. Polymorphic expression of defensins in neutrophils from outbred rats. Infect. Immun. 58, 3899–3902 (1990).
Eisenhauer, P. B. et al. Purification and antimicrobial properties of three defensins from rat neutrophils. Infect. Immun. 57, 2021–2027 (1989).
Eisenhauer, P. B. & Lehrer, R. I. Mouse neutrophils lack defensins. Infect. Immun. 60, 3446–3447 (1992).
Tang, Y. Q., Yuan, J., Miller, C. J. & Selsted, M. E. Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of α-defensins from rhesus macaque leukocytes. Infect. Immun. 67, 6139–6144 (1999).
Zhao, C. et al. RL-37, an α-helical antimicrobial peptide of the rhesus monkey. Antimicrob. Agents Chemother. 45, 2695–2702 (2001).
Cole, A. M. et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl Acad. Sci. USA 99, 1813–1818 (2002).
Munk, C. et al. The θ-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retroviruses 19, 875–881 (2003).
Yasin, B. et al. θ-defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78, 5147–5156 (2004).
Miles, A. M. et al. Nitric oxide synthase in circulating versus extravasated polymorphonuclear leukocytes. J. Leukoc. Biol. 58, 616–622 (1995).
Babior, B. M., Takeuchi, C., Ruedi, J., Gutierrez, A. & Wentworth, P. Jr. Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl Acad. Sci. USA 100, 3031–3034 (2003).
Alam, M. S. et al. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and anti-apoptotic activities. Infect. Immun. 70, 3130–3142 (2002).
Levy, O. Antibiotic proteins of polymorphonuclear leukocytes. Eur. J. Haematol. 56, 263–277 (1996).
Cole, A. M. & Lehrer, R. I. Minidefensins: antimicrobial peptides with activity against HIV-1. Curr. Pharm. Des. 9, 1463–1473 (2003).
Cunliffe, R. N. α-defensins in the gastrointestinal tract. Mol. Immunol. 40, 463–467 (2003).
Durr, M. & Peschel, A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect. Immun. 70, 6515–6517 (2002).
Gallo, R. L. & Nizet, V. Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr. Allergy Asthma Rep. 3, 402–409 (2003).
Skarnes, R. C. & Watson, D. W. Characterization of leukin: an antibacterial factor from leucocytes active against Gram-positive pathogens. J. Exp. Med. 104, 829–845 (1956).
Skarnes, R. C. Leukin, a bactericidal agent from rabbit polymorphonuclear leucocytes. Nature 216, 806–808 (1967).
Hirsch, J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J. Exp. Med. 103, 589–611 (1956).
Hirsch, J. G. Further studies on preparation and properties of phagocytin. J. Exp. Med. 111, 323–337 (1960).
Hirsch, J. G. & Cohn, Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J. Exp. Med. 112, 1005–1014 (1960).
Selsted, M. E., Harwig, S. S., Ganz, T., Schilling, J. W. & Lehrer, R. I. Primary structures of three human neutrophil defensins. J. Clin. Invest 76, 1436–1439 (1985).
Ouellette, A. J. et al. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 62, 5040–5047 (1994).
Huttner, K. M. & Ouellette, A. J. A family of defensin-like genes codes for diverse cysteine-rich peptides in mouse Paneth cells. Genomics 24, 99–109 (1994).
Selsted, M. E., Miller, S. I., Henschen, A. H. & Ouellette, A. J. Enteric defensins: antibiotic peptide components of intestinal host defense. J. Cell Biol. 118, 929–936 (1992).
Jones, D. E. & Bevins, C. L. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315, 187–192 (1993).
Jones, D. E. & Bevins, C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267, 23216–23225 (1992).
Qu, X. D., Lloyd, K. C., Walsh, J. H. & Lehrer, R. I. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect. Immun. 64, 5161–5165 (1996).
Porter, E. M., Bevins, C. L., Ghosh, D. & Ganz, T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 59, 156–170 (2002).
McManaman, J. L. & Bain, D. L. Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. J. Biol. Chem. 277, 21261–21268 (2002).
Morita, Y. et al. Identification of xanthine dehydrogenase/xanthine oxidase as a rat Paneth cell zinc-binding protein. Biochim. Biophys. Acta 1540, 43–49 (2001).
Diamond, G. et al. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc. Natl Acad. Sci. USA 88, 3952–3956 (1991).
Bensch, K. W., Raida, M., Magert, H. J., Schulz-Knappe, P. & Forssmann, W. G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 368, 331–335 (1995).
Garcia, J. R. et al. Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15, 1819–1821 (2001).
Harder, J., Bartels, J., Christophers, E. & Schroder, J. M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276, 5707–5713 (2001).
Schroder, J. M. & Harder, J. Human β-defensin-2. Int. J. Biochem. Cell Biol. 31, 645–651 (1999).
Valore, E. V. et al. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Invest 101, 1633–1642 (1998).
Scheetz, T. et al. Genomics-based approaches to gene discovery in innate immunity. Immunol. Rev. 190, 137–145 (2002).
Schutte, B. C. et al. Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl Acad. Sci. USA 99, 2129–2133 (2002).
Yamaguchi, Y. et al. Identification of multiple novel epididymis-specific β-defensin isoforms in humans and mice. J. Immunol. 169, 2516–2523 (2002).
Liu, L., Zhao, C., Heng, H. H. & Ganz, T. The human β-defensin-1 and α-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics 43, 316–320 (1997).
Bevins, C. L., Jones, D. E., Dutra, A., Schaffzin, J. & Muenke, M. Human enteric defensin genes: chromosomal map position and a model for possible evolutionary relationships. Genomics 31, 95–106 (1996).
Zhao, C. et al. Gallinacin-3, an inducible epithelial β-defensin in the chicken. Infect. Immun. 69, 2684–2691 (2001).
Hill, C. P., Yee, J., Selsted, M. E. & Eisenberg, D. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science 251, 1481–1485 (1991).
Semple, C. A., Rolfe, M. & Dorin, J. R. Duplication and selection in the evolution of primate β-defensin genes. Genome Biol. 4, R31 (2003).
Morrison, G. M., Semple, C. A., Kilanowski, F. M., Hill, R. E. & Dorin, J. R. Signal sequence conservation and mature peptide divergence within subgroups of the murine β-defensin gene family. Mol. Biol. Evol. 20, 460–470 (2003).
Maxwell, A. I., Morrison, G. M. & Dorin, J. R. Rapid sequence divergence in mammalian β-defensins by adaptive evolution. Mol. Immunol. 40, 413–421 (2003).
Hughes, A. L. & Yeager, M. Coordinated amino acid changes in the evolution of mammalian defensins. J. Mol. Evol. 44, 675–682 (1997).
Hughes, A. L. Evolutionary diversification of the mammalian defensins. Cell. Mol. Life Sci. 56, 94–103 (1999).
Wu, E. R., Daniel, R. & Bateman, A. RK-2: a novel rabbit kidney defensin and its implications for renal host defense. Peptides 19, 793–799 (1998).
Valore, E. V. & Ganz, T. Posttranslational processing of defensins in immature human myeloid cells. Blood 79, 1538–1544 (1992).
Leonova, L. et al. Circular minidefensins and post-translational generation of molecular diversity. J. Leukoc. Biol. 70, 461–464 (2001).
Tang, Y. Q. et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 286, 498–502 (1999).
Tran, D. et al. Homodimeric θ-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277, 3079–3084 (2002).
Groisman, E. A., Parra-Lopez, C., Salcedo, M., Lipps, C. J. & Heffron, F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc. Natl Acad. Sci. USA 89, 11939–11943 (1992).
Groisman, E. A. The pleiotropic two-component regulatory system PhoP–PhoQ. J. Bacteriol. 183, 1835–1842 (2001).
Miller, S. I., Pulkkinen, W. S., Selsted, M. E. & Mekalanos, J. J. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58, 3706–3710 (1990).
Eisenhauer, P. B., Harwig, S. S. & Lehrer, R. I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect. Immun. 60, 3556–3565 (1992).
Gunn, J. S. & Miller, S. I. PhoP–PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178, 6857–6864 (1996).
Kristian, S. A., Durr, M., Van Strijp, J. A., Neumeister, B. & Peschel, A. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect. Immun. 71, 546–549 (2003).
Peschel, A. et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410 (1999).
Peschel, A. et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with L-lysine. J. Exp. Med. 193, 1067–1076 (2001).
Staubitz, P., Neumann, H., Schneider, T., Wiedemann, I. & Peschel, A. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 231, 67–71 (2004).
Koprivnjak, T., Peschel, A., Gelb, M. H., Liang, N. S. & Weiss, J. P. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277, 47636–47644 (2002).
Fischer, W. New Targets For New Antimicrobial Agents. (ed. R. Hakenbeck) 47–50 (Spektrum Akademischer, Heidelberg, Germany, 1997).
Poyart, C., Lamy, M. C., Boumaila, C., Fiedler, F. & Trieu-Cuot, P. Regulation of D-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183, 6324–6334 (2001).
Kokryakov, V. N. et al. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 327, 231–236 (1993).
Nakamura, T. et al. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 263, 16709–16713 (1988).
Marrie, T. J., Harding, G. K. & Ronald, A. R. Anaerobic and aerobic urethral flora in healthy females. J. Clin. Microbiol. 8, 67–72 (1978).
Bowie, W. R. et al. Bacteriology of the urethra in normal men and men with non-gonococcal urethritis. J. Clin. Microbiol. 6, 482–488 (1977).
Curnutte, J. T. & Babior, B. M. Effects of anaerobiosis and inhibitors on O2 production by human granulocytes. Blood 45, 851–861 (1975).
Qu, X. D., Harwig, S. S., Oren, A. M., Shafer, W. M. & Lehrer, R. I. Susceptibility of Neisseria gonorrhoeae to protegrins. Infect. Immun. 64, 1240–1245 (1996).
Goodhart, M. E., Ogden, J., Zaidi, A. A. & Kraus, S. J. Factors affecting the performance of smear and culture tests for the detection of Neisseria gonorrhoeae. Sex. Transm. Dis. 9, 63–69 (1982).
Shafer, W. M., Qu, X., Waring, A. J. & Lehrer, R. I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl Acad. Sci. USA 95, 1829–1833 (1998).
Zhao, C., Wang, I. & Lehrer, R. I. Widespread expression of β-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396, 319–322 (1996).
Schnapp, D., Reid, C. J. & Harris, A. Localization of expression of human β-defensin-1 in the pancreas and kidney. J. Pathol. 186, 99–103 (1998).
Hiratsuka, T. et al. Structural analysis of human β-defensin-1 and its significance in urinary tract infection. Nephron 85, 34–40 (2000).
Rubinstein, E. et al. Antibacterial activity of the pancreatic fluid. Gastroenterology 88, 927–932 (1985).
Huttner, K. M., Kozak, C. A. & Bevins, C. L. The mouse genome encodes a single homolog of the antimicrobial peptide human β-defensin 1. FEBS Lett. 413, 45–49 (1997).
Harder, J., Bartels, J., Christophers, E. & Schroder, J. M. A peptide antibiotic from human skin. Nature 387, 861 (1997).
Ong, P. Y. et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347, 1151–1160 (2002).
Nomura, I. et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J. Immunol. 171, 3262–3269 (2003).
Jia, H. P. et al. Discovery of new human β-defensins using a genomics-based approach. Gene 263, 211–218 (2001).
Claeys, S. et al. Human β-defensins and Toll-like receptors in the upper airway. Allergy 58, 748–753 (2003).
Garcia, J. R. et al. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 306, 257–264 (2001).
Sahly, H. et al. Burkholderia is highly resistant to human β-defensin 3. Antimicrob. Agents Chemother. 47, 1739–1741 (2003).
Duits, L. A. et al. Inhibition of hBD-3, but not hBD-1 and hBD-2, mRNA expression by corticosteroids. Biochem. Biophys. Res. Commun. 280, 522–525 (2001).
McDermott, A. M. et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 44, 1859–1865 (2003).
Bissell, J. et al. Expression of β-defensins in gingival health and in periodontal disease. J. Oral Pathol. Med. 33, 278–285 (2004).
Proud, D., Sanders, S. P. & Wiehler, S. Human rhinovirus infection induces airway epithelial cell production of human β-defensin 2 both in vitro and in vivo. J. Immunol. 172, 4637–4645 (2004).
Duits, L. A. et al. Rhinovirus increases human β-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol. Med. Microbiol. 38, 59–64 (2003).
Frohlich, O., Po, C. & Young, L. G. Organization of the human gene encoding the epididymis-specific EP2 protein variants and its relationship to defensin genes. Biol. Reprod. 64, 1072–1079 (2001).
Rodriguez-Jimenez, F. J. et al. Distribution of new human β-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics 81, 175–183 (2003).
Yenugu, S., Hamil, K. G., Radhakrishnan, Y., French, F. S. & Hall, S. H. The androgen-regulated epididymal sperm-binding protein, human β-defensin 118 (DEFB118) (formerly ESC42), is an antimicrobial β-defensin. Endocrinology 145, 3165–3173 (2004).
Zaballos, A., Villares, R., Albar, J. P., Martinez, A. & Marquez, G. Identification on mouse chromosome 8 of new β-defensin genes with regionally specific expression in the male reproductive organ. J. Biol. Chem. 279, 12421–12426 (2004).
Com, E. et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol. Reprod. 68, 95–104 (2003).
Li, P. et al. An antimicrobial peptide gene found in the male reproductive system of rats. Science 291, 1783–1785 (2001).
Rao, J. et al. Cloning and characterization of a novel sperm-associated isoantigen (E-3) with defensin- and lectin-like motifs expressed in rat epididymis. Biol. Reprod. 68, 290–301 (2003).
Zhou, C. X. et al. An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nature Cell Biol. 6, 458–464 (2004).
Fleischmann, J., Selsted, M. E. & Lehrer, R. I. Opsonic activity of MCP-1 and MCP-2, cationic peptides from rabbit alveolar macrophages. Diagn. Microbiol. Infect. Dis. 3, 233–242 (1985).
Fuse, N. et al. Purification and characterization of new anti-adrenocorticotropin rabbit neutrophil peptides (defensins). Eur. J. Biochem. 216, 653–659 (1993).
Ichinose, M. & Sawada, M. A flow cytometric assay reveals a suppression of phagocytosis by rabbit defensin NP-3A in mouse peritoneal macrophages. Microbiol. Immunol. 39, 365–367 (1995).
Tominaga, T. et al. Effects of corticostatin-I on rat adrenal cells in vitro. J. Endocrinol. 125, 287–292 (1990).
Zhu, Q., Bateman, A., Singh, A. & Solomon, S. Isolation and biological activity of corticostatic peptides (anti-ACTH). Endocr. Res. 15, 129–149 (1989).
Zhu, Q. Z., Singh, A. V., Bateman, A., Esch, F. & Solomon, S. The corticostatic (anti-ACTH) and cytotoxic activity of peptides isolated from fetal, adult and tumor-bearing lung. J. Steroid Biochem. 27, 1017–1022 (1987).
Zhu, Q. Z. et al. Isolation and structure of corticostatin peptides from rabbit fetal and adult lung. Proc. Natl Acad. Sci. USA 85, 592–596 (1988).
Shamova, O. V., Lesnikova, M. P., Kokriakov, V. N., Shkhinek, E. K. & Korneva, E. A. The action of defensins on the corticosterone level of the blood and on the immune response in stress. Biull. Eksp. Biol. Med. 115, 646–649 (1993).
Johnson, E. W., Blalock, J. E. & Smith, E. M. ACTH receptor-mediated induction of leukocyte cyclic AMP. Biochem. Biophys. Res. Commun. 157, 1205–1211 (1988).
Blalock, J. E. Propiomelanocortin and the immune–neuroendocrine connection. Ann. NY Acad. Sci. 885, 161–172 (1999).
Johnson, E. W., Hughes, T. K. Jr & Smith, E. M. ACTH receptor distribution and modulation among murine mononuclear leukocyte populations. J. Biol. Regul. Homeost. Agents 15, 156–162 (2001).
Takeuchi, S., Kudo, T. & Takahashi, S. Molecular cloning of the chicken melanocortin 2 (ACTH)-receptor gene. Biochim. Biophys. Acta 1403, 102–108 (1998).
Clarke, B. L. Binding and processing of 125I-ACTH by isolated rat splenic lymphocytes. Biochem. Biophys. Res. Commun. 266, 542–546 (1999).
Tatro, J. B. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation 3, 259–284 (1996).
Befus, A. D. et al. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J. Immunol. 163, 947–953 (1999).
Niyonsaba, F., Someya, A., Hirata, M., Ogawa, H. & Nagaoka, I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur. J. Immunol. 31, 1066–1075 (2001).
Hook, W. A., Tsuji, S. & Siraganian, R. P. Magainin-2 releases histamine from rat mast cells. Proc. Soc. Exp. Biol. Med. 193, 50–55 (1990).
Cross, L. J. et al. Influence of α-helicity, amphipathicity and D-amino acid incorporation on the peptide-induced mast cell activation. Eur. J. Pharmacol. 291, 291–300 (1995).
Di Nardo, A., Vitiello, A. & Gallo, R. L. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 170, 2274–2278 (2003).
Payne, V. & Kam, P. C. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia 59, 695–703 (2004).
Boyce, J. A. Mast cells: beyond IgE. J. Allergy Clin. Immunol. 111, 24–32 (2003).
Huang, C. et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 160, 1910–1919 (1998).
Panyutich, A. V., Szold, O., Poon, P. H., Tseng, Y. & Ganz, T. Identification of defensin binding to C1 complement. FEBS Lett. 356, 169–173 (1994).
Prohaszka, Z. et al. Defensins purified from human granulocytes bind C1q and activate the classical complement pathway like the transmembrane glycoprotein gp41 of HIV-1. Mol. Immunol. 34, 809–816 (1997).
van den Berg, R. H., Faber-Krol, M. C., van Wetering, S., Hiemstra, P. S. & Daha, M. R. Inhibition of activation of the classical pathway of complement by human neutrophil defensins. Blood 92, 3898–3903 (1998).
Wang, W. et al. Activity of α- and θ-defensins against primary isolates of HIV-1. J. Immunol. 173, 515–520 (2004).
Dodds, A. W. Which came first, the lectin/classical pathway or the alternative pathway of complement? Immunobiology 205, 340–354 (2002).
Fujita, T. Evolution of the lectin–complement pathway and its role in innate immunity. Nature Rev. Immunol. 2, 346–353 (2002).
Okrent, D. G., Lichtenstein, A. K. & Ganz, T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am. Rev. Respir. Dis. 141, 179–185 (1990).
Lichtenstein, A. K., Ganz, T., Nguyen, T. M., Selsted, M. E. & Lehrer, R. I. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J. Immunol. 140, 2686–2694 (1988).
Lichtenstein, A., Ganz, T., Selsted, M. E. & Lehrer, R. I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 68, 1407–1410 (1986).
Lehrer, R. I., Lichtenstein, A. K. & Ganz, T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11, 105–128 (1993).
Aarbiou, J. et al. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J. Leukoc. Biol. 72, 167–174 (2002).
Aarbiou, J. et al. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am. J. Respir. Cell Mol. Biol. 30, 193–201 (2004).
Kudriashov, B. A. et al. Effect of defensin on the process of healing of aseptic skin wound and on the permeability of blood vessels. Biull. Eksp. Biol. Med. 109, 391–393 (1990).
Murphy, C. J., Foster, B. A., Mannis, M. J., Selsted, M. E. & Reid, T. W. Defensins are mitogenic for epithelial cells and fibroblasts. J. Cell Physiol. 155, 408–413 (1993).
Sorensen, O. E. et al. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 170, 5583–5589 (2003).
Panyutich, A. & Ganz, T. Activated α2-macroglobulin is a principal defensin-binding protein. Am. J. Respir. Cell Mol. Biol. 5, 101–106 (1991).
Panyutich, A. V., Hiemstra, P. S., van Wetering, S. & Ganz, T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am. J. Respir. Cell Mol. Biol. 12, 351–357 (1995).
Nygaard, S. D., Ganz, T. & Peterson, M. W. Defensins reduce the barrier integrity of a cultured epithelial monolayer without cytotoxicity. Am. J. Respir. Cell Mol. Biol. 8, 193–200 (1993).
Territo, M. C., Ganz, T., Selsted, M. E. & Lehrer, R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 84, 2017–2020 (1989).
Yang, D., Chen, Q., Chertov, O. & Oppenheim, J. J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68, 9–14 (2000).
Yang, D., Biragyn, A., Kwak, L. W. & Oppenheim, J. J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23, 291–296 (2002).
Yang, D. et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528 (1999).
Niyonsaba, F., Ogawa, H. & Nagaoka, I. Human β-defensin-2 functions as a chemotactic agent for tumour necrosis factor-α-treated human neutrophils. Immunology 111, 273–281 (2004).
Biragyn, A. et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science 298, 1025–1029 (2002).
Niyonsaba, F., Iwabuchi, K., Matsuda, H., Ogawa, H. & Nagaoka, I. Epithelial cell-derived human β-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 14, 421–426 (2002).
van Wetering, S. et al. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 272, L888–L896 (1997).
van Wetering, S., Mannesse-Lazeroms, S. P., Van Sterkenburg, M. A. & Hiemstra, P. S. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone. Inflamm. Res. 51, 8–15 (2002).
Spencer, L. T. et al. Role of human neutrophil peptides in lung inflammation associated with α1-antitrypsin deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L514–L520 (2004).
Lin, P. W. et al. Paneth cell cryptdins act in vitro as apical paracrine regulators of the innate inflammatory response. J. Biol. Chem. 279, 19902–19907 (2004).
Cole, A. M. et al. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167, 623–627 (2001).
Yang, D. et al. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J. Leukoc. Biol. 74, 448–455 (2003).
Biragyn, A. et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with non-immunogenic tumor antigens. J. Immunol. 167, 6644–6653 (2001).
Fomicheva, E. E., Pivanovich, I. I., Shamova, O. V. & Nemirovich-Danchenko, E. A. Glucocorticoid hormones in immunomodulating effect of defensins. Ross. Fiziol. Zh. Im I. M. Sechenova 88, 496–502 (2002).
Brogden, K. A. et al. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol. Immunol. 18, 95–99 (2003).
Boyaka, P. N. & McGhee, J. R. Cytokines as adjuvants for the induction of mucosal immunity. Adv. Drug Deliv. Rev. 51, 71–79 (2001).
Oppenheim, J. J., Biragyn, A., Kwak, L. W. & Yang, D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann. Rheum. Dis. 62, S17–S21 (2003).
Zhu, B. D., Feng, Y., Huang, N., Wu, Q. & Wang, B. Y. Mycobacterium bovis bacille Calmette–Guerin (BCG) enhances human β-defensin-1 gene transcription in human pulmonary gland epithelial cells. Acta Pharmacol. Sin. 24, 907–912 (2003).
Lehrer, R. I., Daher, K., Ganz, T. & Selsted, M. E. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J. Virol. 54, 467–472 (1985).
Daher, K. A., Selsted, M. E. & Lehrer, R. I. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60, 1068–1074 (1986).
Sinha, S., Cheshenko, N., Lehrer, R. I. & Herold, B. C. NP-1, a rabbit α-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 47, 494–500 (2003).
Cheshenko, N. & Herold, B. C. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. 83, 2247–2255 (2002).
Spear, P. G., Shieh, M. T., Herold, B. C., WuDunn, D. & Koshy, T. I. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv. Exp. Med. Biol. 313, 341–353 (1992).
Wang, W., Cole, A. M., Hong, T., Waring, A. J. & Lehrer, R. I. Retrocyclin, an antiretroviral θ-defensin, is a lectin. J. Immunol. 170, 4708–4716 (2003).
Broekaert, W. F. et al. Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 31, 4308–4314 (1992).
Huang, R. H. et al. Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv. FEBS Lett. 521, 87–90 (2002).
Van Den Bergh, K. P. et al. Five disulfide bridges stabilize a hevein-type antimicrobial peptide from the bark of spindle tree (Euonymus europaeus L.). FEBS Lett. 530, 181–185 (2002).
Nielsen, K. K., Nielsen, J. E., Madrid, S. M. & Mikkelsen, J. D. Characterization of a new antifungal chitin-binding peptide from sugar beet leaves. Plant Physiol. 113, 83–91 (1997).
Iwanaga, S. et al. Role of hemocyte-derived granular components in invertebrate defense. Ann. NY Acad. Sci. 712, 102–116 (1994).
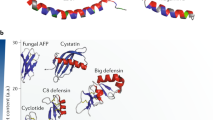
Saito, T. et al. A novel big defensin identified in horseshoe crab hemocytes: isolation, amino acid sequence, and antibacterial activity. J. Biochem. (Tokyo) 117, 1131–1137 (1995).
Kawabata, S. et al. Tachycitin, a small granular component in horseshoe crab hemocytes, is an antimicrobial protein with chitin-binding activity. J. Biochem. (Tokyo) 120, 1253–1260 (1996).
Kawabata, S. et al. cDNA cloning, tissue distribution, and subcellular localization of horseshoe crab big defensin. Biol. Chem. 378, 289–292 (1997).
Beisel, H. G., Kawabata, S., Iwanaga, S., Huber, R. & Bode, W. Tachylectin-2: crystal structure of a specific GlcNAc/GalNAc-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus. EMBO J. 18, 2313–2322 (1999).
Zhang, X. L., Selsted, M. E. & Pardi, A. NMR studies of defensin antimicrobial peptides. 1. Resonance assignment and secondary structure determination of rabbit NP-2 and human HNP-1. Biochemistry 31, 11348–11356 (1992).
Hoover, D. M. et al. The structure of human macrophage inflammatory protein-3α/CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human β-defensins. J. Biol. Chem. 277, 37647–37654 (2002).
Nakashima, H., Yamamoto, N., Masuda, M. & Fujii, N. Defensins inhibit HIV replication in vitro. AIDS 7, 1129 (1993).
Zhang, L. et al. Contribution of human α-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298, 995–1000 (2002).
Mackewicz, C. E., Ortega, H. & Levy, J. A. Effect of cytokines on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell. Immunol. 153, 329–343 (1994).
Cohen, J. AIDS research. Mystery anti-HIV factor unmasked? Science 297, 2188 (2002).
Zhang, L., Lopez, P., He, T., Yu, W. & Ho, D. D. Retraction of an interpretation. Science 303, 467 (2004).
Vella, C. & Daniels, R. S. CD8+ T-cell-mediated non-cytolytic suppression of human immuno-deficiency viruses. Curr. Drug Targets Infect. Disord. 3, 97–113 (2003).
Chang, T. L., Francois, F., Mosoian, A. & Klotman, M. E. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from α-defensin-1 HIV inhibition. J. Virol. 77, 6777–6784 (2003).
Trabattoni, D. et al. Human α-defensin in HIV-exposed but uninfected individuals. J. Acquir. Immune. Defic. Syndr. 35, 455–463 (2004).
Mars, W. M. et al. Inheritance of unequal numbers of the genes encoding the human neutrophil defensins HP-1 and HP-3. J. Biol. Chem. 270, 30371–30376 (1995).
Hollox, E. J., Armour, J. A. & Barber, J. C. Extensive normal copy number variation of a β-defensin antimicrobial-gene cluster. Am. J. Hum. Genet. 73, 591–600 (2003).
King, A. E., Fleming, D. C., Critchley, H. O. & Kelly, R. W. Differential expression of the natural antimicrobials, β-defensins 3 and 4, in human endometrium. J. Reprod. Immunol. 59, 1–16 (2003).
King, A. E., Critchley, H. O. & Kelly, R. W. Innate immune defences in the human endometrium. Reprod. Biol. Endocrinol. 1, 116 (2003).
Circo, R., Skerlavaj, B., Gennaro, R., Amoroso, A. & Zanetti, M. Structural and functional characterization of hBD-1(Ser35), a peptide deduced from a DEFB1 polymorphism. Biochem. Biophys. Res. Commun. 293, 586–592 (2002).
Matsushita, I. et al. Genetic variants of human β-defensin-1 and chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun. 291, 17–22 (2002).
Nguyen, T. X., Cole, A. M. & Lehrer, R. I. Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides 24, 1647–1654 (2003).
Trabi, M., Schirra, H. J. & Craik, D. J. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from rhesus macaque leukocytes. Biochemistry 40, 4211–4221 (2001).
Weiss, T. M. et al. Two states of cyclic antimicrobial peptide RTD-1 in lipid bilayers. Biochemistry 41, 10070–10076 (2002).
Schibli, D. J. et al. The solution structures of the human β-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J. Biol. Chem. 277, 8279–8289 (2002).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author holds over 20 patents on various antimicrobial peptides, including several on defensins. In line with university policy, these patents have been assigned to the University of California.
Supplementary information
Related links
Rights and permissions
About this article
Cite this article
Lehrer, R. Primate defensins. Nat Rev Microbiol 2, 727–738 (2004). https://doi.org/10.1038/nrmicro976
Issue Date:
DOI: https://doi.org/10.1038/nrmicro976
This article is cited by
-
The role of lipopolysaccharides in diabetic retinopathy
BMC Ophthalmology (2022)
-
Co-AMPpred for in silico-aided predictions of antimicrobial peptides by integrating composition-based features
BMC Bioinformatics (2021)
-
Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice
Virology Journal (2020)
-
Effects of Dietary Supplementation of Recombinant Plectasin on Growth Performance, Intestinal Health and Innate Immunity Response in Broilers
Probiotics and Antimicrobial Proteins (2020)
-
RP-HPLC-ESI-IT Mass Spectrometry Reveals Significant Variations of the Human Salivary Protein Profile Associated with Predominantly Antibody Deficiencies
Journal of Clinical Immunology (2020)