Key Points
-
Polio is set to be the second viral disease to be eradicated owing to the truly extraordinary efforts of a consortium led by the World Health Organization. Only a few pockets of infection remain in the world, a major global achievement.
-
Once the circulating wild-type virus is eradicated, there is no justification for continuing vaccination.
-
Cessation of vaccination will not be straightforward because the vaccine used is a live virus that can regain virulence in vaccinees. Cases of vaccine-associated polio have been documented for many years, and several small outbreaks have been caused by strains that are derived from the vaccine strains in specific circumstances. Some immunocompromised individuals can excrete virulent virus for many years.
-
Possible strategies for cessation of vaccination include stopping the use of any vaccine, switching to the use of inactivated vaccines or a combination of both of these strategies. These strategies depend on assumptions for success. Simply stopping vaccination assumes that the vaccine strains will die out, whereas the use of inactivated vaccines will require the manufacture of vaccine under conditions where the virus cannot escape. Inactivated vaccines may not prevent person to person spread under all conditions.
-
Once eradicated, poliovirus could still re-emerge, for example from laboratory stocks or old clinical samples, or other more speculative sources, including bioterror events. Vaccine stockpiles are required to cope with these potential outcomes but, ironically, vaccine production could also be a potential source of future outbreaks.
-
New tools to deal with poliovirus that are designed based on our current understanding of poliovirus biology are indeed possible, but are difficult to develop.
Abstract
The poliovirus eradication programme coordinated by the World Health Organization mainly involves immunization with a live attenuated vaccine. Only six countries — India, Pakistan, Afghanistan, Egypt, Nigeria and Niger — still have endemic poliovirus. To tackle recent outbreaks in India and Nigeria, eradication strategies have been refocused. It was hoped that complete eradication of wild-type poliovirus would be achieved by the end of 2003, but during 2004 there have been setbacks to polio immunization in Nigeria, and the number of poliomyelitis cases has increased. It still seems possible that the virus could be eradicated — but should vaccination continue when that goal has been achieved?
Similar content being viewed by others
Main
Poliovirus has been studied for many years and the mechanisms of replication and the structure of the capsid are well understood (Box 1). Poliovirus pathogenesis is not as well defined, but the events following infection have been understood in general terms since the early twentieth century (Box 2). The broad history of the eradication programme and its current status can be obtained from the World Health Organization (WHO) polio eradication web site (see the online links box).
Polio was recognized as a disease of developed countries in the twentieth century, particularly in the 1950s when epidemics involving thousands of cases occurred regularly in the United States and Europe. In the 1970s, lameness surveys showed that polio was also a significant disease in developing countries. Smallpox was eradicated in 1979, and the WHO identified six other diseases that seemed suited to control by worldwide vaccination — diphtheria, tetanus, pertussis, measles, tuberculosis and polio. For each of these diseases there is a reliable and stable vaccine against the causative agent. In addition, for each of these diseases, there is no major animal reservoir — unlike, for example, yellow fever — which makes control of the disease more likely through immunization of the human population. These vaccination programmes formed the core of the expanded programme on immunization (EPI), which was established in 1974.
Progress in the eradication of poliovirus was rather slow, except in the region overseen by the Pan American Health Organization (PAHO), which includes North, Central and South America (Timeline). In 1985, the PAHO committed itself to the eradication of polio by 1990 on the basis of their progress at that time. The strategies used, and why they were required, are discussed below, but in 1988, the World Health Assembly, the governing body of the WHO, committed the organization to the global eradication of poliovirus by the year 2000 (41st World Health Assembly Resolution 41-28). Several donors have funded the eradication programme — including the United Nations Childrens Fund UNICEF, the WHO, the Centers for Disease and Control (CDC), various member states and Rotary International — and this commitment to the programme has had an important effect on its progress. The sums involved are substantial; Rotary International alone raised US $247 million in 1988, and it is anticipated that the programme will need US $975 million for 2003–20051.

The eradication programme has used the live attenuated vaccines that were developed by Sabin from circulating wild-type strains of poliovirus (Box 3). There are three vaccine strains (one derived from each wild-type poliovirus serotype). These vaccine strains are excreted by recipients for several weeks after infection. Oral (Sabin) polio vaccine (OPV) has been used in mass vaccination programmes, in which very large populations are immunized over a short period of time, such as a few days, whether the individuals are due for immunization or not. These campaigns include national immunization days (NIDS), in which millions of children in a country are immunized in a single day, and sub-national immunization days (SNIDS), in which an area smaller than a whole country is covered2. NIDS and SNIDS are designed to abruptly reduce the number of susceptible individuals and therefore interrupt any transmission of the virus in the population. The objective is to prevent all transmissions, even silent transmission that does not result in disease; the virus then dies out. Eradication of the infectious agent is the only means by which an infectious disease can be eradicated.
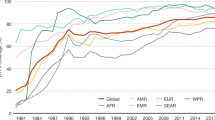
Routine immunization against poliovirus is a different strategy that is focused on protecting the individual, typically when a particular age is reached. For example, in the United Kingdom OPV is given when a child reaches two, three and four months of age, or in response to a particular individual need, such as a visit to a region where poliovirus is endemic. Routine immunization specifically protects individuals, although circulation of poliovirus can be interrupted by this method using specific strategies — for example, high coverage of routine vaccination. Unfortunately, in many parts of the developing world routine immunization coverage is very low. Routine immunization is also far less effective, in principle, at blocking transmission in tropical countries. This is likely to be due partly to the difficulties associated with storage of the vaccine in hot climates, where it is likely to lose potency unless expensive storage systems are in place, partly to the level of coverage that can be consistently achieved over a period of several years, and partly to the epidemiology of poliovirus circulation. In temperate climates, poliovirus transmission is highly seasonal, occurring mostly in the summer. Routine immunization in the winter months can reduce the number of susceptible individuals to such an extent that virus transmission is restricted in the season of peak transmission so that the virus eventually dies out. By contrast, in tropical climates, poliovirus transmission occurs throughout the year, albeit with some seasonal peaks, so if routine immunization strategies are followed it is more likely that an individual will become infected with the wild-type poliovirus before that individual is vaccinated. The pool of susceptible individuals is therefore little reduced in tropical countries3 unless routine immunization with high coverage is aggressively targeted at the very young or newborn. Routine immunization is used globally in conjunction with NIDS and SNIDS and additional 'mopping-up' strategies, such as intensive immunization — for example, by house to house visits that target areas that are otherwise difficult to reach for social, geographic or other reasons. Although the combination of these strategies has eradicated poliovirus from most of the world (Fig. 1), transmission still occurs in six countries — India, Pakistan, Afghanistan, Egypt, Niger and Nigeria. In 2003, the most significant outbreaks were located in India and Nigeria, but in 2004 significant resistance to immunization programmes had developed in Nigeria, and poliovirus began to re-emerge in areas that had previously been poliovirus-free (see the polio eradication web site in the online links box). It is likely that the considerable efforts that have been made to get the programme back on track have been successful, but the events in Nigeria illustrate the complexity of massive long-term, global public-health programmes.
With the eradication of the wild-type poliovirus underway we now need to consider whether it is possible to stop vaccination without environmental vaccine-derived strains regaining the virulence of wild-type strains, and the associated risks of transmissability and the ability to cause paralysis. Mechanisms by which poliovirus could persist, and possible counter-measures that could be used should this virus re-emerge, are discussed in this review.
Vaccine-derived poliomyelitis cases
Vaccine-associated paralytic poliomyelitis. Cases of vaccine-associated paralytic poliomyelitis (VAPP) have been reported in vaccine recipients of OPV and their immediate contacts, and occur at low frequencies. Primary vaccinees can become paralysed at 1 per 750,000 recipients of the vaccine4. The rate of paralysis after two vaccine doses is 1 per 12 million recipients4. VAPP frequencies are comparable in all countries in which they have been measured4,5,6,7,8. VAPP patients display a typical poliomyelitis pathology, and the viruses that are isolated from affected individuals are genetically very similar to the vaccine strains used. VAPP develops due to the genetic instability of the poliovirus when it replicates in the gut. The number of cases of VAPP in recipients and contacts is approximately the same. VAPP cases are rare, and cases in secondary contacts (known contacts of known contacts of vaccinees) have not been reported. Nonetheless, transmission must take place, with or without causing disease, or circulating vaccine-derived polioviruses (cVDPVs) could not be isolated.
Circulating vaccine-derived polioviruses. Several outbreaks of poliomyelitis have occurred in regions where poliovirus has been eradicated. Affected countries include Haiti and the Dominican Republic (which together form the island of Hispaniola)9, Egypt10, the Philippines and Madagascar11. Determination of either the complete sequence of the viral RNA genome isolated from infected individuals, or only the region of the genome that encodes VP1 (capsid protein), enables the relationship between poliovirus isolates to be determined. Sequence analyses indicate that the viruses that are responsible for these outbreaks have evolved from one of the live vaccine strains used in the eradication programmes. In addition, as the rate of drift in the sequence of the poliovirus RNA genome is constant at approximately 3% per year, if silent mutations are considered, or approximately 1% per year if all mutations are taken into account, irrespective of serotype or strain, it is possible to determine when two related strains diverged. The extensive changes throughout the sequence showed that the vaccine-derived strains had been circulating unrecognized for at least two years. Routine immunization coverage of the population in the affected regions was low or patchy, although it is not clear how low the coverage or how large the poorly covered population has to be before cVDPVs become a serious hazard. In Haiti, the coverage was about 30%, whereas in the Philippines the figure was closer to 80% (Ref. 11). Immunized individuals, most probably children, excreting a vaccine strain of the virus, are thought to have transmitted poliovirus to susceptible, non-immunized individuals. Routine immunization programmes with poor coverage might persist for several years, giving ample opportunity for cVDPVs to appear.
All cVDPV strains that have been characterized so far are composed of the structural proteins of one of the vaccine strains, whereas the rest of the virus derives from so-far unidentified enteroviruses from the same virus group as poliovirus12 (Box 1). It is thought that recombination between the vaccine strain and an enterovirus is responsible for emergence of the cVDPV strains that have been identified so far. For each cVDPV outbreak that has been analysed the recombinant enterovirus partner is different9,10,11, and in Haiti, the Dominican Republic and Madagascar there was evidence for multiple recombination events with different enterovirus partners9,11.
cVDPVs are defined by the criterion that they differ from the relevant vaccine strain by more than 1%, taking all nucleotide positions into account, and have therefore been in circulation for more than a year. This distinguishes them from most vaccine-derived poliovirus isolates that are excreted by vaccine recipients, which are also likely to have accumulated some sequence drift during the process of replication in the vaccinee. Although viruses that have been circulating for less than a year, and therefore differ by less than 1% in sequence, exist, these viruses cannot be identified. In addition, vaccine-derived viruses with significant sequence drift are occasionally isolated from sewage or healthy individuals in the absence of any outbreak or disease13,14,15. These cVDPVs are being transmitted silently. It has been claimed that cVDPVs occur even in regions where vaccine coverage is maintained at a high level15. On the one hand, this seems unlikely as the vaccine is able to eradicate circulating wild-type poliovirus, as shown by the observation that the wild-type virus has not yet re-emerged in any area of the world that has been certified as free of poliovirus. On the other hand, the coverage that is required to prevent the development of cVDPVs is not known, and silently circulating strains of the virus have been identified in regions where vaccination was still taking place13,14. It is also obvious that a non-immunized population of the appropriate size in a region with otherwise very high coverage might be sufficient to allow cVDPVs to arise; however, the population size that is required is unknown11.
Immune-deficient vaccine-derived polioviruses. Although vaccine-derived polioviruses are excreted for several weeks after vaccination in healthy individuals, excretion of the vaccine strain sometimes takes place for prolonged periods of time in patients that have defective humoral immunity (also known as immunodeficient long-term excretors). Immune-deficient vaccine-derived polioviruses (iVDPVs) were first identified when immunodeficient patients were deliberately given live polio vaccine in the late 1960s in an attempt to stimulate immunity to poliovirus, or otherwise prevent infection, in individuals who were at particular risk at a time when wild-type poliovirus was a major hazard in the United Kingdom16. Fewer than twenty cases of iVDVP have been described17,18. iVDPV can be distinguished from VAPP by continued excretion of poliovirus by a single individual, and by the sequence of the virus which, although related to the vaccine strain, shows significant sequence drift. Some cases of iVDPV have been identified serendipitously and were not associated with paralysis, whereas in other cases sequencing of the excreted iVDPV genome indicated that the virus was excreted over a long period of time before paralysis occurred19. In these examples the patient probably remained healthy because they were also treated with intravenously administered immunoglobulin, which confines poliovirus to the gut (Box 2). Similar amounts of poliovirus are excreted by both healthy vaccinees and iVDPV patients (about 4–5 log10 infectious units per gram of stool) Poliovirus that is excreted by iVPDV individuals is usually, but not always, virulent in animal models20. Although there have been no reports of iVPDV patients infecting people with whom they come into contact, this could be due to good standards of hygiene in those countries in which iVDPV cases have been described. In contrast with outbreak cVDPVs, iVDPVs have not as yet been generated by recombination between a vaccine strain and an unknown enterovirus. At present, no successful treatment is available to prevent virus excretion by iVDPV patients21, although in many cases virus excretion ceases spontaneously.
The occurrence of VAPP, iVDPVs and cVDPVs indicates that vaccine strains can regain virulence, transmit between individuals with low or high efficiency, or persist in an individual without being recognized for weeks to years. Once the wild-type virus is eradicated, the only cause of paralytic polio disease will be vaccine-derived strains.
Even if there are no competing health needs, it is unlikely that immunization could be maintained indefinitely against a non-existent disease at a level that is sufficient to prevent vaccine-derived viruses evolving to cause epidemics. This is due to the cost of administering the vaccine and to the demands made on an infrastructure that could be used for other purposes compared with the trivial likely benefit to the individual. Cessation of vaccination therefore seems to be the only option, but the use of the live vaccine makes this problematic. This dilemma is a clear threat to the successful complete eradication of polio.
Strategies for the cessation of vaccination
Abrupt cessation. Poliovirus is labile in the environment and persists only by infecting new hosts. Apart from the rare cases of iVDPVs, excretion of virus by an infected individual is not thought to persist for more than six months22. If vaccination was stopped, there would be no VAPP and new cVDPVs or iVDPVs would not emerge.
If cessation of vaccination was accompanied by a mass immunization programme there would be few susceptible individuals, and the relatively short period of excretion in normal individuals should allow less time for the selection of cVDPVs than a continuing programme with low coverage. Theoretically, the vaccine strain should die out more rapidly than susceptible hosts can be born. The success of such a strategy would need to be evaluated by thorough monitoring of virus excretion — for example, through the examination of sewage or other environmental samples13,22,23, possibly for several years — and would involve abandoning routine immunization against poliovirus altogether.
The persistent excretion of virus poses a different problem. There is no reliable treatment available for existing cases of iVDPV infection that can prevent virus excretion. As the precise number of iVDPV cases is not known, iVDPV infection represents an undefined reservoir of vaccine-derived poliovirus. So far, no outbreaks have been attributed to iVDPV, and it is possible that they do not pose a risk of transmission, although this seems unlikely. The iVDPV strains could be evaluated to determine their environmental impact and potential transmissibility, while protecting immediate contacts with inactivated polio vaccine (IPV). This could prove difficult in developing countries, but cases of iVDPV have not been reported in these areas.
In short, abrupt cessation of vaccination could eradicate poliovirus; however, this conclusion is based on assumptions that remain uncertain.
Use of the inactivated polio vaccine. Discontinuing the use of OPV and instead using the IPV (Box 3) will prevent VAPP in both vaccine recipients and their immediate contacts, and stop the generation of new cases of iVDPV or cVDPV. Many developed countries, including those of Europe and North America, have already begun to use the IPV rather then the OPV for this reason (see polio eradication web site in the online links box). As a global strategy however, this could prove difficult to implement, owing to the current cost of the IPV (which is about ten times that of the OPV if administered on its own) and potential supply problems (at present, the IPV is manufactured on a smaller scale than the OPV). The main problem however, is that good coverage is required, and it is not feasible to use IPV in NIDS programmes (which have good coverage) because it is administered by injection, which requires greater skill than OPV administration. IPV could be used in routine immunization programmes, possibly in combination with other vaccines such as diptheria, tetanus and pertussis, but at present, the coverage achieved in such programmes can be very low. In addition, it would be necessary to prevent transmission of poliovirus. IPV has been shown to prevent transmission in developed countries such as Holland and the Scandinavian countries. This is thought to have been possible because, although IPV might not prevent infection of the gut, it does prevent spread of the virus from the gut to the throat by viraemia (Box 2). As the main route of transmission in developed countries with high levels of hygiene is thought to be respiratory rather than faecal–oral, IPV effectively prevents spread of the virus. It is not known whether IPV use would prevent transmission in countries with reduced levels of hygiene — where there is an increased chance of faecal–oral transmission — and there could be extensive silent spread of the virus with outbreaks of disease in unimmunized individuals.
Combination strategy. The strategy that is most likely to be selected following the declaration of the eradication of poliovirus will depend almost entirely on social, practical and economic circumstances. Developed countries are likely to continue immunizing against poliovirus as they have done for many years in the absence of endemic disease, and will most likely use IPV, probably in combination with other vaccines used in paediatric vaccination programmes. This will be justified owing to the uncertainty surrounding poliovirus eradication and the concerns raised by the identification of long-term excreters of the virus in developed countries. The additional cost of IPV in such programmes is likely to be acceptable. By contrast, developing countries are unlikely to be able to afford IPV; currently much of the polio vaccine used in these countries is purchased by international aid agencies and following poliovirus eradication this supply of vaccine is likely to stop. In many developing countries, other health problems, such as HIV, tuberculosis and malaria, are more important public-health hazards than polio. So, developing countries are likely to cease vaccinating abruptly, whereas developed countries could continue to vaccinate with IPV. Therefore, the global post-eradication strategy will be a combination of these two approaches.
Re-emergence of poliovirus
It is possible that poliovirus could re-emerge after it has been eradicated either because wild-type strains might persist undetected or because the vaccine strains could evolve and circulate in a population. Either wild-type virus or vaccine-derived strains could 'escape' from clinical or research laboratories, or from vaccine stocks that are held by manufacturers. Clinical samples might contain wild-type poliovirus — for example, samples that are used to investigate gastrointestinal disease in a region that has endemic poliovirus at the time when the specimens were taken. It has been shown that laboratory stocks of other viruses can become contaminated with poliovirus24. OPV is a source of poliovirus infections and currently the origin of limited outbreaks of disease. IPV is manufactured by growing wild-type poliovirus to high titre in cell culture before inactivation with formalin (Box 3). The WHO has investigated several cases in which poliovirus has escaped from an IPV-manufacturing facility25,26. Production of IPV from the live vaccine strains could be less hazardous, and might be attractive to manufacturers starting new production processes. Existing vaccine manufacturers may however, find the logistics of integrating a new virus strain into their existing complex vaccine production too difficult. It is likely to require many extensive clinical trials to show that a new IPV is as effective as previous vaccines and does not interfere with the other components (such as diphtheria, tetanus and pertussis) that might be administered in the same combination product. They are therefore likely to choose to produce IPV in high containment. Even production of IPV from vaccine strains will eventually have to be carried out using high-containment laboratory facilities, and the level of containment that would be considered adequate is a matter for debate. Biological safety level 3 (BSL3) containment is generally thought to be suitable for the immediate future, although it is unclear whether this level will be considered suitable over a longer timescale. Poliovirus could also be deliberately re-introduced — for example, by bioterrorism — once herd immunity declines. Finally, there are plausible scenarios in which a poliovirus could evolve from a related virus, such as a coxsackievirus; however, these scenarios are speculative27.
In response to these concerns it has been proposed that stocks of OPV vaccine should be maintained for an unspecified period of time for use in the event of re-emergence of poliovirus28,29. OPV would be chosen because this vaccine has previously been successfully used to stop transmission during epidemics28,29,30. The stockpiled vaccine should be monovalent — vaccine derived from a single serotype — because the vaccine will be used after mass vaccination has ceased. Use of the trivalent OPV would re-introduce all three serotypes into the population, which would clearly be undesirable. How, and when, the vaccine stocks would be used is unclear. One problem with this strategy is that there could be a delay between the occurrence of a suspected poliomyelitis case and verification by isolation of poliovirus, probably at least 60 days on the basis of current expectations31. In the intervening 60-day period, any circulating poliovirus could disseminate world-wide, and could result in a requirement for a global vaccination day, which is unlikely to be practicable.
The development of new tools to fight polio
It is possible that the existing tools will be sufficient to eradicate poliovirus completely if they are used in an appropriate way. Researchers and clinicians have extensive experience with the existing live vaccines, which, despite the problems that they might present over a long period of time, are very safe and effective. Any change in vaccine usage poses scientific, clinical, manufacturing and logistical problems, and must be carefully planned and monitored. The problems that could arise are illustrated by the experience with the alternative vaccine strain USOL-D-Bac.
Satisfactorily attenuated batches of the type 3 Sabin vaccine strain are difficult to produce, and the properties of the virus change rapidly after excretion by vaccinees — within one week strains become more neurovirulent in animal models32. The USOL-D-Bac vaccine strain was an attractive alternative that was under investigation in the late 1960s. It was highly immunogenic and was more stable in vaccinees than the Sabin type 3 strain33. However, a few months after a trial of the USOL-D-Bac and the Sabin strains in Poland there was an outbreak of poliomyelitis that was subsequently shown to be due to strains that were derived from the USOL-D-Bac vaccine strain34,35. No reported cases of polio were attributed to the clinical trial of the Sabin strain. The epidemiological circumstances in Poland were unusual because the authorities had been using live type 1 and 2 vaccines together with inactivated type 3 vaccine, but the results of the trial severely inhibited any subsequent developments in live vaccines, owing to the possible safety risks.
The scope of the eradication effort requires global administration, and it will be extremely difficult to change current practices, even if there is overwhelming scientific evidence of the benefit. Currently, OPV is used in campaigns such as NIDS, and both IPV and OPV are used in routine immunization programmes. All polio vaccines that are in current use are trivalent, and include strains of each of the three serotypes. Even the introduction of a monovalent vaccine to deal with an outbreak of a single serotype after eradication will be difficult. At present, monovalent vaccines are not licensed, although one monovalent vaccine was used in some countries (such as South Africa) until relatively recently. Licensing is likely to require trials of safety and efficacy and is expensive and time-consuming, so manufacturers are unlikely to attempt to license a vaccine if there is no guarantee that there will be a market for the vaccine.
At the same time however, it is clear that the current vaccines present potentially serious problems for the final stages of the eradication of poliovirus. The uncoordinated and patchy cessation of vaccination or reintroduction of vaccination with OPV, caused significant problems in the 1960s in Russia with the appearance of virulent transmissible strains15. In this case, vaccination against poliovirus was stopped in certain areas of Belarus where polio had been controlled. Two final mass immunization programmes were carried out, but two years later, poliovirus was isolated from individuals. It was shown by sequence analyses and assessing mutations that the viruses that were isolated had been circulating for at least two years and therefore had not been introduced from other parts of Belarus where vaccination programmes had continued. One isolate was a recombinant virus that was comparable with the cVDPVs that have been isolated in the recent past. Serological studies indicated that many people had been infected with poliovirus in the absence of vaccination, implying that the virus was still circulating.
There is a danger that this type of infection event could be repeated throughout the world following cessation of vaccination. Poliovirus is one of the most well-studied and best-understood viruses. It is worth considering whether this knowledge should be exploited to devise a new vaccine or intervention therapy that is suitable for the final stages of eradication, while acknowledging that, in the absence of an overwhelming need, it will be very difficult to bring any vaccine or therapy to a usable form for scientific, commercial, clinical and legal reasons.
Considerations for polio vaccine design
The ideal polio vaccine would not cause VAPP, iVDPV or cVDPV, would be phenotypically stable and would be unable to transmit between individuals. In practice, a vaccine strain that did not transmit between individuals and that rarely reverted to virulence might be sufficient. The existing live vaccines undergo extensive genetic changes during replication in the normal recipient. An attenuating mutation identified in the 5′ non-coding region of the virus (Box 1) reverts rapidly during virus replication in the human gut36. The mutation affects the stability of a base-paired structure that is involved in the initiation of protein synthesis, possibly in a tissue-specific manner. By manipulating the base-pairing in this region, it is possible to generate a structure of the same thermodynamic stability that requires multiple mutations to revert to the wild-type and should therefore be more stable. Viruses that have this structure are attenuated, as predicted, and are stable during passage in vitro37. Other approaches to generate more stable poliovirus strains have involved exchange of the 5′ non-coding region for that of a rhinovirus, a closely related virus with a different tissue tropism38.
Other mutations in the capsid regions also revert, or are suppressed at high frequency, but over a longer period of time while the existing vaccine strains replicate in the gut of recipients. The suppression of these mutations can be extremely subtle39 and therefore difficult to counter through manipulation of the virus genome. Introducing stable mutations into structural proteins is likely to be more difficult than stabilizing base-paired nucleic acid structures, as the rules that govern nucleic acid stability are simpler. However, the tissues in which poliovirus grows, and the disease that it causes, are determined largely by the structure of the virus particle, and therefore by the capsid region of the genome. So, recombination with another enterovirus could remove an attenuating mutation outside the capsid region while maintaining the virus as a poliovirus that is able to cause polio. In principle, mutations in the capsid proteins are the only mutations that can produce a stable, attenuated poliovirus that is incapable of reversion. For example, mutations in the 5′ non-coding region could be removed by recombination, while maintaining the ability of the virus to cause polio.
It might be possible to devise a vaccine that would not depend on the growth of live virus. Although peptide and subunit vaccines against poliovirus have not been successful owing to the conformational nature of the antigenic structure of the virus, it is possible that DNA vaccines might be devised40. This strategy has been used to develop foot and mouth disease vaccines (which are targeted against a picornavirus) with limited success41.
Vaccine development is an empirical science and is dependent on clinical trials. Using a molecular approach it is possible to devise plausible vaccine candidates and to monitor their performance more readily and precisely than in the past, especially in terms of the stability of a live attenuated strain. With the difficulties that are associated with introducing a new polio vaccination strategy, a new vaccine against poliovirus is unlikely to be developed unless there is an overwhelming need. On the other hand, the research that is required to identify vaccine candidates is relatively inexpensive and it would be a useful strategy against poliovirus re-emergence.
Concluding remarks
Eradication of poliomyelitis disease will only be successful if the poliovirus is eradicated. The main strategy that has been used so far is the live attenuated poliovirus vaccine developed by Sabin, which is known to revert to produce a virulent and transmissible virus — albeit at low frequency. Eradication of vaccine strains is also necessary for disease prevention. This is complicated by the absence of intervention therapies other than the existing licensed vaccines, the inherent difficulties in changing widely adopted vaccination practices and the lack of a commercial incentive to develop new polio therapies with the prospect of disease eradication. The simplest approach would be to use either OPV or IPV.
If poliovirus re-emerged, it is assumed that the available vaccines would prove safe and efficacious but, although this is probably true, it is not certain. First, live vaccines would re-introduce poliovirus to the environment. Second, it might not be possible to use IPV effectively. Finally, it is conceivable that the live vaccine could have different safety issues if recipients lacked maternal antibody to poliovirus, which would be the case in a naive population, or are completely seronegative and unprotected from revertant strains that are generated by virus replication in the gut. The alternative to stopping vaccination — a permanent vaccine programme — does not seem likely.
Therefore, it might be prudent to consider other options after eradication of poliovirus, such as new vaccines or other intervention strategies that are based on an understanding of poliovirus pathogenesis and ecology. Ideally, this should be carried out before poliovirus is eradicated owing to the increasing difficulties of working with the virus after the disease has been eliminated.
Failure to address the concerns surrounding the cessation of vaccination and its aftermath could result in re-emergence of poliovirus and reverse the global achievement of the eradication programme.
References
Aylward, R. B. et al. Disease eradication as a public health strategy: a case study of poliomyelitis eradication. Bull. World Health Organ. 78, 285–297 (2000). Discusses the global eradication programme.
Hull, H. F., Ward, N. A., Hull, B. R., Milstien, J. B. & de Quadros, C. Paralytic poliomyelitis: seasoned strategies, disappearing disease. Lancet 343, 1331–1337 (1994).
Nathanson, N. & Martin, J. R. The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity and disappearance. Am. J. Epidemiol. 110, 672–692 (1979).
Nkowane, B. U., Wassilak, S. G. & Orenstein, W. A. Vaccine-associated paralytic poliomyelitis in the United States: 1973 through 1984. JAMA 257, 1335–1340 (1987). Assesses the frequency of cases of vaccine-associated poliomyelitis (VAPP).
Andrus, J. K., Strebel, P. M., de Quadros, C. & Olive, J. M. Risk of vaccine-associated paralytic poliomyelitis in Latin America, 1989–1991. Bull. World Health Organ. 73, 33–40 (1995).
Strebel, P. M. et al. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus associated disease. Clin. Inf. Dis. 14, 568–579 (1992).
Kohler, K. A. et al. Compatible poliomyelitis cases in India during 2000. Bull. World Health Organ. 81, 2–9 (2003).
Joce, R., Wood, D., Brown, D. & Begg, N. Paralytic poliomyelitis in England and Wales 1985–1991. Brit. Med. Bull. 305, 79–87 (1992).
Kew, O. et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296, 356–359 (2002).
Yang, C. -F. et al. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 77, 8366–8377 (2003).
Kew, O. M. et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 82, 16–23 (2004). A thorough review of outbreaks caused by circulating vaccine-derived polioviruses.
Stanway, G. et al. Taxonomy of the picornaviridae: species designation and three new genera. [online] (European Study Group on the Molecular Biology of Picornaviruses, Italy, 2000).
Shulman, L. M. et al. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral polio vaccine strain isolated from sewage in Israel. J. Clin Microbiol. 38, 3729–3734 (2000).
Cherkasova, E. et al. Microarray analysis of evolution of RNA viruses: evidence of circulation of virulent highly divergent vaccine-derived polioviruses. Proc. Natl Acad. Sci. USA 100, 9398–9403 (2003).
Korotkova, E. A. et al. Retrospective analysis of a local cessation of vaccination against poliomyelitis: a possible scenario for the future. J. Virol. 77, 12460–12465 (2003).
MacCallum, F. O. Hypogammaglobulinaemia in the United Kingdom, VII. The role of humoral antibodies in protection against and recovery from bacterial and virus infections in hypogammaglobulinaemia. Spec. Rep. Ser. Med. Res. Counc. 310, 72–85 (1971).
Minor, P. D. Characteristics of poliovirus strains from long-term excretors with primary immunodeficiencies. Dev. Biol. (Basel) 105, 75–80 (2001).
Halsey, N. A. et al. Search for poliovirus carriers among people with primary immune deficiency diseases in the United States, Mexico, Brazil and the United Kingdom. Bull. World Health Organ. 82, 3–8 (2004). A good summary of cases of poliomyelitis arising due to immune-deficient long-term excreters of poliovirus.
Bellmunt, A. et al. Evolution of poliovirus type 1 during 5.5 years of prolonged enteral replication in an immune deficient patient. Virology 265, 178–184 (1999).
Martin, J., Dunn, G., Hull, R., Patel, V. & Minor, P. D. Evoluation of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J. Virol. 74, 3001–3010 (2000).
MacLennan, C. A. et al. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet (in the press).
Poyry, T., Stenvik, M. & Hovi, T. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl. Environ. Microbiol. 54, 371–374 (1988).
El Bassioni, L. et al. Prolonged detection of indigenous wild polioviruses in sewage from communities in Egypt. Am. J. Epidemiol. 158, 807–815 (2003).
Davies, M. et al. Poliovirus type 1 in working stocks of typed human rhinoviruses. Lancet 361, 1187–1188 (2003).
Mulda, M. N. et al. Genetic analysis of wild-type poliovirus importation into the Netherlands (1979–1995). J. Infect. Dis. 176, 617–624 (1997).
Minor, P. D. Biosafety consequences of eradication of wild-type polioviruses. Lancet 358, 166–168 (2001). Discusses the problems associated with containment of poliovirus after eradication.
Rieder, E. et al. Will the polio niche remain vacant? Dev. Biol. (Basel) 105, 111–122 (2001).
Carcares, V. M. & Sutter, R. W. Sabin monovalent oral poliovaccines: review of past experiences and their potential use after polio eradication. Clin. Infect. Dis. 33, 531–541 (2001).
Fine, P. E. M., Oblapenko, G. & Sutter, R. E. Polio control after certification: major issues outstanding. Bull. World Health Organ. 82, 47–52 (2004).
Fine, P. E. & Carneiro, I. A. Transmissibility and persistence of oral poliovaccine viruses: implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 150, 1001–1021 (1999). This article outlines the epidemiological considerations that are key to the global eradication of poliovirus.
Kojouharana, M. et al. Importation and circulation of poliovirus in Bulgaria in 2001. Bull. World Health Organ. 81, 476–481 (2003).
WHO Consultative Group. Evidence on the safety and efficacy of live poliomyelitis vaccines currently in use, with special reference to type 3 poliovirus. Bull. World Health Organ. 40, 925–945 (1969).
Elbert, L. B. et al. Comparative study of two vaccine strains of type 3 poliovirus (USOL-D-bac and Leon 12a1b). Acta Virol. 11, 89–99 (1967).
Kostrezewski, J., Kulesza, A. & Abgarowicz, A. The epidemic of type 3 poliomyelitis in Poland in 1968. Epidemiol. Rev. 24, 89–103 (1970).
Martin, J., Ferguson, G., Wood, D. & Minor, P. D. The vaccine origin of the 1968 epidemic of type 3 poliomyelitis in Poland. Virology 278, 42–49 (2000).
Dunn, G., Begg, N. T., Cammack, N. & Minor, P. D. Virus excretion and mutation by infants following primary vaccination with live oral poliovaccine from two sources. J. Med. Virol. 32, 92–95 (1990).
Macadam, A. J. et al. Live-attenuated strains of improved stability. Dev. Biol. (Basel) 105, 179–187 (2001).
Gromeier, M., Lachmann, S., Rosenfeld, M. R., Gutin, P. H. & Wimmer, E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl Acad. Sci. USA 97, 6803–6808 (2000).
Minor, P. D. The molecular biology of poliovaccines. J. Gen. Virol. 73, 3065–3077 (1992). Introduction to the virology of polio and polio vaccines.
Minor, P. D. in Picornaviruses: Current Topics in Microbiology and Immunology Vol. 161 (ed. Racaniello, V. R.) 121–154 (Springer, 1990).
Cedillo-Barron, L., Foster-Cuevas, M., Belsham, G. J., Lefevre, F. & Parkhouse, R. M. Induction of a protective response in swine vaccinated with DNA encoding foot and mouth disease virus empty capsid proteins and the 3D RNA polymerase. J. Gen. Virol. 82, 1713–1724 (2001).
Minor, P. D. in Viral Pathogenesis (eds Nathanson, N. et al.) 555–574 (Lippincott Raven Philadelphia, New York, 1997). An excellent review of poliovirus pathogenesis.
Sabin, A. B. & Boulger, L. History of Sabin attenuated poliovirus oral live vaccine strains. J. Biol. Stand. 1, 115–118 (1973).
van Wezel, A. L., van Steenis, G., van der Marel, P. & Osterhaus, A. D. M. E. Inactivated poliovirus vaccine: current production methods and new developments. Rev. Infect. Dis. 6, S335–S340 (1984).
Mendelsohn, C.L., Wimmer, E., Racaniello, V.R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56, 855–865 (1989).
Schmid, M. & Wimmer, E. IRES-controlled protein synthesis and genome replication of poliovirus. Arch. Virol. 9, S279–S289 (1994).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Infectious Disease Information
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Minor, P. Polio eradication, cessation of vaccination and re-emergence of disease. Nat Rev Microbiol 2, 473–482 (2004). https://doi.org/10.1038/nrmicro906
Issue Date:
DOI: https://doi.org/10.1038/nrmicro906
This article is cited by
-
Using short-message-service notification as a method to improve acute flaccid paralysis surveillance in Papua New Guinea
BMC Public Health (2016)
-
Evaluation of AFP surveillance indicators in polio-free Ghana, 2009–2013
BMC Public Health (2014)
-
Engineering attenuated virus vaccines by controlling replication fidelity
Nature Medicine (2008)
-
A deader vaccine?
Nature Medicine (2008)
-
Rotavirus vaccines: recent developments and future considerations
Nature Reviews Microbiology (2007)