Key Points
-
Behavioural and physiological processes of numerous organisms are controlled by a circadian clock. Of these, the simplest model system to uncover the workings of these rhythms is the unicellular cyanobacterium, Synechococcus elongatus PCC 7942.
-
Automated detection of circadian expression from luciferase gene fusions allows for large-scale mutant hunts to identify necessary components of the bacterial clock.
-
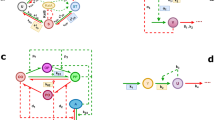
A locus of three genes — kaiA, kaiB, and kaiC — was found to encode the core of the clock. Mutation of any of these genes results in altered circadian rhythms, including arrhythmicity and short or long periods.
-
Each Kai protein interacts with itself and with each of the other Kai proteins.
-
Functions have been assigned to KaiA and KaiB on the basis of their effects on the phosphorylation state of KaiC — KaiA stimulates KaiC autophosphorylation and KaiB abrogates the positive effect of KaiA.
-
CikA and LdpA interpret environmental input as timing cues to reset the clock to local time or to fine-tune the period of the rhythm under varying light intensities, respectively.
-
The SasA protein closely associates with the central Kai proteins and transduces temporal information to cellular processes that are under circadian control.
-
The Kai proteins and SasA are co-purified in large macromolecular complexes during the night. The abundance of each protein, as well as the size of the complex, changes with the light/dark cycle.
Abstract
For more than three billion years, the organisms on this planet have known, like Little Orphan Annie, that “The sun'll come out tomorrow”, and many have honed their biochemistry to exploit this knowledge. The cyanobacteria have had ample time to fashion a suitable timepiece, as they are among the oldest inhabitants of the earth. For these organisms, light is food, and it is a nutrient that shows up at the same time every day. Not surprisingly, cyanobacteria have learned to arrange their days around dinnertime.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
deMairan, J. J. Observation botanique (1729).
Harmer, S. L., Panda, S. & Kay, S. A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 17, 215–253 (2001).
Mitsui, A., Kumazawa, S., Takahashi, A., Ikemoto, H. & Arai, T. Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. Nature 323, 720–722 (1986).
Huang, T. -C., Tu, J., Chow, T. J. & Chen, T. -H. Circadian rhythm of the prokaryote Synechococcus sp. RF-1. Plant Physiol. 92, 531–533 (1990).
Chen, T. -H., Chen, T. -L., Hung, L. -M. & Huang, T. -C. Circadian rhythm in amino acid uptake by Synechococcus RF-1. Plant Physiol. 97, 55–59 (1991).
Sweeney, B. M. & Borgese, M. B. A circadian rhythm in cell division in a prokaryote, the cyanobacterium Synechococcus WH7803. J. Phycol. 25, 183–186 (1989).
Pittendrigh, C. S. in Handbook of Behavioral Neurobiology: Biological Rhythms (ed. Aschoff, J.) 57–80, 95–124 (Plenum Press, New York, 1981).
Dunlap, J. C., Loros, J. J. & DeCoursey, P. J. Chronobiology: Biological Timekeeping (Sinauer Associates, Sunderland, Massachusetts, 2003).
Millar, A. J., Short, S. R., Chua, N. -H. & Kay, S. A. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4, 1075–1087 (1992).
Young, M. W. & Kay, S. A. Time zones: a comparative genetics of circadian clocks. Nature Rev. Genet. 2, 702–715 (2001).
Allada, R., Emery, P., Takahashi, J. S. & Rosbash, M. Stopping time: the genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci. 24, 1091–1119 (2001).
Emery, P., So, W. V., Kaneko, M., Hall, J. C. & Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998).
Griffin, E. A. Jr, Staknis, D. & Weitz, C. J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286, 768–771 (1999).
Okamura, H. et al. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science 286, 2531–2534 (1999).
He, Q., Cheng, P., Yang, Y., Yu, H. & Liu, Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 22, 4421–4430 (2003).
Schauer, A. et al. Visualizing gene expression in time and space in the filamentous bacterium Streptomyces coelicolor. Science 240, 768–772 (1988).
Kay, S. A. Shedding light on clock controlled cab gene transcription in higher plants. Semin. Cell Biol. 4, 81–86 (1993).
Golden, S. S. Light-responsive gene expression in cyanobacteria. J. Bacteriol. 177, 1651–1654 (1995).
Kondo, T. et al. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc. Natl Acad. Sci. USA 90, 5672–5676 (1993).
Liu, Y. et al. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 9, 1469–1478 (1995).
Kondo, T. et al. Circadian clock mutants of cyanobacteria. Science 266, 1233–1236 (1994).
Ishiura, M. et al. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281, 1519–1523 (1998). The discovery of the kai genes and their function of central oscillator in the cyanobacterial clock.
Lorne, J., Scheffer, J., Lee, A., Painter, M. & Miao, V. P. Genes controlling circadian rhythm are widely distributed in cyanobacteria. FEMS Microbiol. Lett. 189, 129–133 (2000).
Dvornyk, V., Vinogradova, O. & Nevo, E. Long-term microclimatic stress causes rapid adaptive radiation of kaiABC clock gene family in a cyanobacterium, Nostoc linckia, from “Evolution Canyons” I and II, Israel. Proc. Natl Acad. Sci. USA 99, 2082–2087 (2002).
Dvornyk, V., Vinogradova, O. & Nevo, E. Origin and evolution of circadian clock genes in prokaryotes. Proc. Natl Acad. Sci. USA 100, 2495–2500 (2003).
Aoki, S., Kondo, T. & Ishiura, M. Circadian expression of the dnaK gene in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 177, 5606–5611 (1995).
Rocap, G. et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424, 1042–1047 (2003).
Rippka, R., Waterbury, J. B. & Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 100, 419–436 (1974).
Nishimura, H. et al. Mutations in KaiA, a clock protein, extend the period of circadian rhythm in the cyanobacterium Synechococcus elongatus PCC 7942. Microbiology 148, 2903–2909 (2002).
Darlington, T. K. et al. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280, 1599–1603 (1998).
Crosthwaite, S. K., Dunlap, J. C. & Loros, J. J. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276, 763–769 (1997).
Cheng, P., Yang, Y. & Liu, Y. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc. Natl Acad. Sci. USA 98, 7408–7413 (2001).
Glossop, N. R., Lyons, L. C. & Hardin, P. E. Interlocked feedback loops within the Drosophila circadian oscillator. Science 286, 766–768 (1999).
Aronson, B. D., Johnson, K. A., Loros, J. J. & Dunlap, J. C. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science 263, 1578–1584 (1994).
Xu, Y., Mori, T. & Johnson, C. H. Cyanobacterial circadian clockwork: roles of KaiA, KaiB and the kaiBC promoter in regulating KaiC. EMBO J. 22, 2117–2126 (2003).
Iwasaki, H., Taniguchi, Y., Ishiura, M. & Kondo, T. Physical interactions among circadian clock proteins KaiA, KaiB and KaiC in cyanobacteria. EMBO J. 18, 1137–1145 (1999).
Nishiwaki, T., Iwasaki, H., Ishiura, M. & Kondo, T. Nucleotide binding and autophosphorylation of the clock protein KaiC as a circadian timing process of cyanobacteria. Proc. Natl Acad. Sci. USA 97, 495–499 (2000).
Mori, T. & Johnson, C. H. Circadian programming in cyanobacteria. Semin. Cell Dev. Biol. 12, 271–8 (2001).
Mori, T. et al. Circadian clock protein KaiC forms ATP-dependent hexameric rings and binds DNA. Proc. Natl Acad. Sci. USA 99, 17203–17208 (2002).
Hayashi, F. et al. ATP-induced hexameric ring structure of the cyanobacterial circadian clock protein KaiC. Genes Cells 8, 287–296 (2003). References 39 and 40 show that homotypic interactions of KaiC result in a hexamer and that this formation is necessary for clock function.
Iwasaki, H., Nishiwaki, T., Kitayama, Y., Nakajima, M. & Kondo, T. KaiA-stimulated KaiC phosphorylation in circadian timing loops in cyanobacteria. Proc. Natl Acad. Sci. USA 99, 15788–15793 (2002).
Xu, Y., Mori, T. & Johnson, C. H. Circadian clock-protein expression in cyanobacteria: rhythms and phase setting. EMBO J. 19, 3349–3357 (2000).
Williams, S. B., Vakonakis, I., Golden, S. S. & Li Wang, A. C. Structure and function from the circadian clock protein KaiA of Synechococcus elongatus: A potential clock input mechanism. Proc. Natl Acad. Sci. USA 99, 15357–15362 (2002). The first use of combined structural and biochemical data to denote function to a clock protein.
Kitayama, Y., Iwasaki, H., Nishiwaki, T. & Kondo, T. KaiB functions as an attenuator of KaiC phosphorylation in the cyanobacteria circadian clock system. EMBO J. 22, 1–8 (2003).
Stock, A., Robinson, V. & Goudreau, P. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000).
Matsushika, A., Makino, S., Kojima, M. & Mizuno, T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant Cell Physiol. 41, 1002–1012 (2000).
Strayer, C. et al. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289, 768–771 (2000).
Schmitz, O., Katayama, M., Williams, S. B., Kondo, T. & Golden, S. S. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289, 765–768 (2000). This study describes a key component required for resetting the clock to environmental input signals.
Asato, Y. Toward an understanding of cell growth and the cell division cycle of unicellular photoautotrophic cyanobacteria. Cell. Mol. Life Sci. 60, 663–687 (2003).
Mutsuda, M., Michel, K. P., Zhang, X., Montgomery, B. L. & Golden, S. S. Biochemical properties of CikA, an unusual phytochrome-like histidine protein kinase that resets the circadian clock in Synechococcus elongatus PCC 7942. J. Biol. Chem. 278, 19102–19110 (2003).
Katayama, M., Kondo, T., Xiong, J. & Golden, S. S. ldpA encodes an iron-sulfur protein involved in light-dependent modulation of the circadian period in the cyanobacterium Synechococcus elongatus PCC 7942. J. Bacteriol. 185, 1415–1422 (2003).
Aschoff, J. in Handbook of Behavioral Neurobiology: Biological Rhythms (ed. Aschoff, J.) 81–93 (Plenum Press, New York, 1981).
Nagaya, M., Aiba, H. & Mizuno, T. Cloning of a sensory-kinase-encoding gene that belongs to the two-component regulatory family from the cyanobacterium Synechococcus sp. PCC 7942. Gene 131, 119–124 (1993).
Iwasaki, H. et al. A KaiC-interacting sensory histidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell 101, 223–233 (2000). This work identifies an essential component of the circadian output pathway that interacts directly with proteins in the central oscillator.
Klewer, D. A., Williams, S. B., Golden, S. S. & Li Wang, A. C. Sequence-specific resonance assignments of the N-terminal, 105-residue KaiC-interacting domain of SasA, a protein necessary for a robust circadian rhythm in Synechococcus elongatus. J. Biomol. NMR 24, 77–78 (2002).
Kageyama, H., Kondo, T. & Iwasaki, H. Circadian formation of clock protein complexes by KaiA, KaiB, KaiC and SasA in cyanobacteria. J. Biol. Chem. 278, 2388–2395 (2003). This paper provides evidence of clock-related proteins forming multimeric complexes in a circadian pattern.
Taniguchi, Y. et al. Two KaiA-binding domains of cyanobacterial circadian clock protein KaiC. FEBS Lett. 496, 86–90 (2001).
Edery, I., Zwiebel, L. J., Dembinska, M. E. & Rosbash, M. Temporal phosphorylation of the Drosophila PERIOD protein. Proc. Natl Acad. Sci. USA 91, 2260–2264 (1994).
Liu, Y., Loros, J. & Dunlap, J. C. Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc. Natl Acad. Sci. USA 97, 234–239 (2000).
Liu, Y., Golden, S. S., Kondo, T., Ishiura, M. & Johnson, C. H. Bacterial luciferase as a reporter of circadian gene expression in cyanobacteria. J. Bacteriol. 177, 2080–2086 (1995).
Millar, A. J. & Kay, S. A. Circadian control of cab gene transcription and mRNA accumulation in Arabidopsis. Plant Cell 3, 541–550 (1991).
Ouyang, Y., Andersson, C. R., Kondo, T., Golden, S. S. & Johnson, C. H. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl Acad. Sci. USA 95, 8660–8664 (1998). This study shows a biological advantage of possessing a circadian rhythm that closely matches the length of external cycles.
Kondo, T. et al. Circadian rhythms in rapidly dividing cyanobacteria. Science 275, 224–227 (1997).
Mori, T. & Johnson, C. H. Independence of circadian timing from cell division in cyanobacteria. J. Bacteriol. 183, 2439–2444 (2001).
Mori, T., Binder, B. & Johnson, C. H. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc. Natl Acad. Sci. USA 93, 10183–10188 (1996).
Johnson, J. E., Lackner, L. L. & de Boer, P. A. Targeting of DMinC/MinD and DMinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings. J. Bacteriol. 184, 2951–2962 (2002).
Judd, E. M., Ryan, K. R., Moerner, W. E., Shapiro, L. & McAdams, H. H. Fluorescence bleaching reveals asymmetric compartment formation prior to cell division in Caulobacter. Proc. Natl Acad. Sci. USA 100, 8235–8240 (2003).
Wadhams, G. H. et al. TlpC, a novel chemotaxis protein in Rhodobacter sphaeroides, localizes to a discrete region in the cytoplasm. Mol. Microbiol. 46, 1211–1221 (2002).
Ben-Yehuda, S., Rudner, D. Z. & Losick, R. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299, 532–536 (2003).
Acknowledgements
We gratefully acknowledge the contributions of past and current members of the Golden lab, and in particular thank E. M. Clerico for the data in Figure. 4. We also thank J. L. Ditty, N. Ivleva, H. Iwasaki, M. Katayama, T. Kondo, A. LiWang, M. Sugita, J. Vakonakis, and S. B. Williams for sharing unpublished information, and P. A. Youderian for initiating the functional genomics project. Our work on S. elongatus circadian rhythms and functional genomics is supported by grants from the National Institutes of Health, National Science Foundation and Department of Energy to S. S. G.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez
LocusLink
SwissPort
FURTHER INFORMATION
Glossary
- OSCILLATOR
-
In circadian biology, it is a subset of genes and their protein products that are sufficient to produce a circadian rhythm of activity.
- WALKER A MOTIF
-
A motif (GXXXGKT), where X is any amino acid residue, that is involved in nucleotide-binding of many ATP-requiring enzymes.
- 'TWO-COMPONENT' REGULATORY SYSTEMS
-
A signal-transduction system using two components — a histidine protein kinase (HPK) and a response regulator (RR) — to sense and respond to external stimuli. HPKs autophosphorylate at a histidyl residue after stimulation and transfer that phosphoryl group to a cognate RR at its aspartyl residue to induce a conformational change in the regulatory domain, which, in turn, activates an associated domain.
- PSEUDO-RECEIVER
-
A protein with sequence or structural similarity to the receiver domains of response regulator proteins, but that lacks the aspartyl residue necessary for accepting a phosphoryl group.
- GAF MOTIF
-
A ubiquitous motif, found in sensory proteins of both prokaryotic and eukaryotic cells, that performs a multitude of functions, including bilin lyase activity in bacteriophytochromes of cyanobacteria.
Rights and permissions
About this article
Cite this article
Golden, S., Canales, S. Cyanobacterial circadian clocks — timing is everything. Nat Rev Microbiol 1, 191–199 (2003). https://doi.org/10.1038/nrmicro774
Issue Date:
DOI: https://doi.org/10.1038/nrmicro774
This article is cited by
-
Frequency of change determines effectiveness of microbial response strategies
The ISME Journal (2023)
-
Association of healthy beverage index with circadian rhythm and quality of sleep among overweight and obese women: a cross-sectional study
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
High protein copy number is required to suppress stochasticity in the cyanobacterial circadian clock
Nature Communications (2018)
-
Methods for enhancing cyanobacterial stress tolerance to enable improved production of biofuels and industrially relevant chemicals
Applied Microbiology and Biotechnology (2018)
-
Minimal tool set for a prokaryotic circadian clock
BMC Evolutionary Biology (2017)