Key Points
-
The loss of immune tolerance results in the breakdown of immune homeostasis and the appearance of exacerbated inflammatory conditions and autoimmune diseases.
-
Several mechanisms act together to ensure self-tolerance, including clonal deletion, anergy, ignorance and exhaustion, effector T-cell and regulatory T-cell balance and cytokine deviation. Identifying factors that regulate these processes is crucial for the development of new therapies for inflammatory and autoimmune diseases.
-
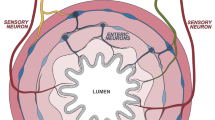
Certain neuropeptides and hormones are produced in response to exacerbated inflammatory responses. Some of these neuropeptides, including the vasoactive intestinal peptide, α-melanocyte-stimulating hormone, urocortin, adrenomedullin and cortistatin, have emerged as endogenous anti-inflammatory factors that are involved in regulating immune tolerance, acting at several levels.
-
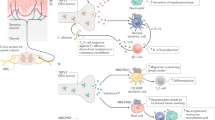
Anti-inflammatory neuropeptides bind to specific receptors that are expressed in immune cells, activate the cyclic AMP–protein kinase A pathway, and downregulate various transcription factors that are involved in the inflammatory response and in activating T cells.
-
These neuropeptides reduce the production of pro-inflammatory cytokines, chemokines and free radicals by macrophages, dendritic cells (DCs) and microglia, and inhibit the activation and differentiation of T helper 1 (TH1) cells.
-
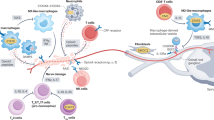
These neuropeptides induce the emergence of regulatory T cells, especially under autoimmune conditions, involving several mechanisms. Neuropeptides directly induce the generation of CD4+CD25+ regulatory T cells in the periphery from the CD4+CD25− T-cell repertoire. In addition, neuropeptides generate tolerogenic DCs that have the capacity to induce interleukin-10-producing regulatory T cells.
-
The capacity of these neuropeptides to regulate the inflammatory response, the activation of autoreactive T cells and the generation of regulatory T cells, makes them attractive candidates for treating several inflammatory and autoimmune disorders. These neuropeptides are therapeutically beneficial in experimental models of sepsis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and type 1 diabetes.
-
Cellular therapies that are based on the use of regulatory T cells or tolerogenic DCs generated with neuropeptides are effective in treating various autoimmune diseases and transplantation.
Abstract
The induction of antigen-specific tolerance is essential to maintain immune homeostasis, control autoreactive T cells, prevent the onset of autoimmune diseases and achieve tolerance of transplants. Inflammation is a necessary process for eliminating pathogens, but can lead to serious deleterious effects in the host if left unchecked. Identifying the endogenous factors that control immune tolerance and inflammation is a key goal in the field of immunology. In the last decade, various neuropeptides that are produced by immune cells with potent anti-inflammatory actions were found to participate in the maintenance of tolerance in different immunological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wraith, D. C., Nicolson, K. S. & Whitlet, N. T. Regulatory CD4+ T cells and the control of autoimmune disease. Curr. Opin. Immunol. 16, 695–701 (2004).
Wood, K. J. & Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nature Rev. Immunol. 3, 199–210 (2003).
Said, S. I. & Mutt, V. Polypeptide with broad biological activity: isolation from small intestine. Science 169, 1217–1218 (1970).
Said, S. I. & Rosenberg, R. N. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science 192, 907–908 (1976).
Hadley, M. E. & Haskell-Luevano, C. The proopiomelanocortin system. Ann. NY Acad. Sci. 885, 1–21 (1999).
Abdel-Malek, Z. A. Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell. Mol. Life Sci. 58, 434–441 (2001).
Bellinger, D. L. et al. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides 18, 1139–1149 (1997).
Delgado, M. & Ganea, D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J. Immunol. 166, 2907–2912 (2001). The first study describing the production of VIP by antigen-stimulated T H 2-type CD4+ and type 2 CD8+ T cells, and suggesting that, from an immunological point of view, a neuropeptide could be considered a cytokine.
Blalock, J. E. Proopiomelanocortin and the immune-neuroendocrine connection. Ann. NY Acad. Sci. 885, 161–172 (1999).
Star, R. A. et al. Evidence of autocrine modulation of macrophage nitric oxide synthase by α-melanocyte-stimulating hormone. Proc. Natl Acad. Sci. USA 92, 8016–8020 (1995).
Harmar, A. et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 50, 265–270 (1998).
Delgado, M., Pozo, D. & Ganea, D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol. Rev. 56, 249–290 (2004).
Neumann Andersen, G. et al. MC1 receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin. Exp. Immunol. 126, 441–446 (2001).
Catania, A. et al. The neuropeptide α-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides 17, 675–679 (1996).
Becher, E. et al. Human peripheral blood-derived dendritic cells express functional melanocortin receptor MC-1R. Ann. NY Acad. Sci. 885, 188–195 (1999).
Taherzadeh, S. et al. α-MSH and its receptors in regulation of tumor necrosis factor-α production by human monocyte/macrophages. Am. J. Physiol. 276, R1289–R1294 (1999)
Getting, S. J., Gibbs, L., Clark, A. J., Flower, R. J. & Perretti, M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J. Immunol. 162, 7446–7453 (1999).
Buggy, J. J. Binding of α-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem. J. 331, 211–216 (1998).
Taylor, A. & Namba, K. In vitro induction of CD25+CD4+ regulatory T cells by the neuropeptide α-melanocyte stimulating hormone (α-MSH). Immunol. Cell Biol. 79, 358–367 (2001).
Medzhitov, R. & Janeway, C. A. Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 5–298 (1997).
Lipton, J. M. & Catania, A. Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol. Today 18, 140–145 (1997). An outstanding and comprehensive review of the significance of α-MSH in immunomodulation.
Delgado, R. et al. Melanocortin peptides inhibit production of proinflammatory cytokines and nitric oxide by activated microglia. J. Leukoc. Biol. 63, 740–745 (1998).
Bhardwaj, R. S. et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J. Immunol. 156, 2517–2521 (1996).
Delgado, M., Munoz-Elias, E. J., Gomariz, R. P. & Ganea, D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNγ synthesis by T cells. J. Neuroimmunol. 96, 167–181 (1999).
Delgado, M. et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNF production by macrophages: in vitro and in vivo studies. J. Immunol. 162, 2358–2367 (1999). The first study describing the potential anti-inflammatory action of VIP.
Delgado, M., Muñoz-Elías, E. J., Gomariz, R. P. & Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J. Immunol. 162, 1707–1716 (1999).
Delgado, M., Muñoz-Elías, E. J., Gomariz, R. P. & Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-κB and IFN regulatory factor 1 activation. J. Immunol. 162, 4685–4696 (1999).
Hernanz, A., Tato, E., De la Fuente, M., de Miguel, E. & Arnalich, F. Differential effects of gastrin-releasing peptide, neuropeptide Y, somatostatin and vasoactive intestinal peptide on interleukin-1β, interleukin-6 and tumor necrosis factor-α production by whole blood cells from healthy young and old subjects. J. Neuroimmunol. 71, 25–30 (1996).
Martinez, C. et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide modulate endotoxin-induced IL-6 production by murine peritoneal macrophages. J. Leukoc. Biol. 63, 591–601 (1998).
Delgado, M. & Ganea, D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J. Immunol. 167, 966–975 (2001).
Luger, T. A., Scholzen, T. E., Brzoska, T. & Bohm, M. New insights into the functions of α-MSH and related peptides in the immune system. Ann. NY Acad. Sci. 994, 133–140 (2003).
Delgado, M., Reduta, A., Sharma, V. & Ganea, D. VIP/PACAP oppositely affect immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity of CD4+ T cells. J. Leukoc. Biol. 75, 1122–1130 (2004). References 31 and 32 report the effect of α-MSH and VIP in the co-stimulatory activity of APCs on the clonal expansion of effector T cells through the regulation of the co-stimulatory molecules CD80 and CD86.
Gomariz, R. P. et al. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J. Leukoc. Biol. 78, 491–502 (2005).
Sarkar, A., Sreenivasan, Y. & Manna, S. K. α-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced biological responses by downregulating CD14 from macrophages. FEBS Lett. 553, 286–294 (2003).
Delgado, M. et al. Shedding of membrane-bound CD14 from lipopolysaccharide-stimulated macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J. Neuroimmunol. 99, 61–71 (1999). References 33–35 and 88 report that α-MSH and VIP downregulate the inflammatory response by inhibiting the initial steps of signalling through TLR2, TLR4 and CD14.
Delgado, M., Leceta, J., Gomariz, R. P. & Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by upregulating B7.2 expression. J. Immunol. 163, 3629–3635 (1999).
Voice, J. K., Dorsam, G., Lee, H., Kong, Y. & Goetzl, E. J. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 15, 2489–2496 (2001).
Goetzl, E. J. et al. Enhanced delayed-type hypersensitivity and diminished immediate type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. Proc. Natl Acad. Sci. USA 98, 13854–13859 (2001).
Voice, J. K. et al. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J. Immunol. 170, 308–314 (2003). An important study showing the role of VIP that is endogenously produced by lymphocytes in the regulation of the T H 1/T H 2 balance in vivo.
Ranger, A. M., Das, M. P., Kuchroo, V. K. & Glimcher, L. M. B7-2 (CD86) is essential for the development of IL-4 producing T cells. Int. Immunol. 8, 1549–1560 (1996).
Delgado, M., Gonzalez-Rey, E. & Ganea, D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J. 18, 1453–1455 (2004).
Delgado, M., Leceta, J. & Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J. 16, 1844–1846 (2002).
Voice, J. et al. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J. Immunol. 172, 7289–7296 (2004). References 41–43 describe the different mechanisms involved in the preferential induction of T H 2 responses by VIP.
Shevach, E. M. CD4+CD25+ suppressor T cells: more questions than answers. Nature Rev. Immunol. 2, 816–822 (2002). An outstanding review that summarizes recent findings in the field of regulatory T cells and gives an outlook for future directions.
Delgado, M., Chorny, A., Gonzalez-Rey, E. & Ganea, D. Vasoactive intestinal peptide generates CD4+CD25+regulatory T cells in vivo. J. Leukoc. Biol. 78, 1327–1338 (2005).
Namba, K., Kitaichi, N., Nishida, T. & Taylor, A. W. Induction of regulatory T cells by the immunomodulating cytokines α-melanocyte stimulating hormone and transforming growth factor-β2. J. Leukoc. Biol. 72, 946–952 (2002).
Nishida T. & Taylor, A. W. Specific aqueous humor factors induce activation of regulatory T cells. Invest. Ophthalmol. Vis. Sci. 40, 2268–2274 (1999). References 19 and 45–47 are the first studies describing the induction of regulatory T cells by neuroimmune mediators.
Gonzalez-Rey, E., Fernandez-Martin, A., Chorny, A. & Delgado, M. Vasoactive intestinal peptide induces CD4+CD25+ regulatory T cells with therapeutic effect on collagen-induced arthritis. Arthritis Rheum. 54, 864–876 (2006).
Zheng, S. G., Wang, J. H., Gray, J. D., Soucier, H. & Horwitz, D. A. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J. Immunol. 172, 5213–5221 (2004).
Hackstein, H. & Thomson, A. W. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nature Rev. Immunol. 4, 24–34 (2004). An outstanding review of the potential for the use of DCs as a cell-based therapy for the treatment of different immune disorders.
Gonzalez-Rey, E., Chorny, A., Fernandez-Martin, A., Ganea, D. & Delgado, M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood 107, 3632–3638 (2006).
Delgado, M., Gonzalez-Rey, E. & Ganea, D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J. Immunol. 175, 7311–7324 (2005).
Oki, Y. & Sasano, H. Localization and physiological roles of urocortin. Peptides 25, 1745–1749 (2004).
Gravanis, A. & Margioris, A. N. The corticotropin-releasing factor (CRF) family of neuropeptides in inflammation: potential therapeutic applications. Curr. Med. Chem. 12, 1503–1512 (2005).
Kageyama, K. et al. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology 140, 5651–558 (1999).
Correa, S. G., Riera, C. M., Spiess, J. & Bianco, I. D. Modulation of the inflammatory response by corticotropin-releasing factor. Eur. J. Pharmacol. 319, 85–90 (1997).
Agelaki, S., Tsatsanis, C., Gravanis, A. & Margioris, A. N. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect. Immun. 70, 6068–6074 (2002).
Venihaki, M., Dikkes, P., Carrigan, A & Karalis, K. P. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J. Clin. Invest. 108, 1159–1166 (2001).
La Fleur, S. E., Wick, E. C., Idumalla, P. S., Gray, E. F. & Bhargava, A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc. Natl Acad. Sci. USA 102, 7647–7652 (2005).
Poliak, S. et al. Stress and autoimmunity: the neuropeptides corticotropin-releasing factor and urocortin suppress encephalomyelitis via effects on both the hypothalamic–pituitary–adrenal axis and the immune system. J. Immunol. 158, 5751–5756 (1997). The first study showing the immunomodulatory effect of urocortin in an experimental model of autoimmune disease.
Gonzalez-Rey, E., Chorny, A., Robledo, G. & Delgado, M. Urocortin and adrenomedullin prevent lethal endotoxemia by downregulating the inflammatory response. Am. J. Pathol. 168, 1921–1930 (2006).
Gonzalez-Rey, E., Fernandez-Martin, A., Chorny, A. & Delgado, M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn's disease. Gut 55, 824–832 (2006).
Agnello, D. et al. Corticosteroid-independent inhibition of tumor necrosis factor production by the neuropeptide urocortin. Am. J. Physiol. Endocrinol. Metab. 275, e757–e762 (1998). References 61–63 report the anti-inflammatory action of urocortin through the inhibition of the production of a wide range of inflammatory cytokines by macrophages and monocytes.
Tsatsanis, C. et al. Urocortin 1 and urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett. 579, 4259–4264 (2005).
Chatzaki, E. et al. Urocortin in human gastric mucosa: relationship to inflammatory activity. J. Clin. Endocrinol. Metab. 88, 478–483 (2003).
Hauger, H. L. et al. International union of pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol. Rev. 55, 21–26 (2003).
Hinson, J. P., Kapas, S. & Smith, D. M. Adrenomedullin, a multifunctional regulatory peptide. Endocrin. Rev. 21, 138–167 (2000).
Elsasser, T. H. & Kahl, S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc. Res. Tech. 57, 120–129 (2002).
Kubo, A. et al. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J. Biol. Chem. 273, 16730–16738 (1998).
Wong, L. Y., Cheung, B. M., Li, Y. Y. & Tang, F. Adrenomedullin is both proinflammatory and antiinflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology 146, 1321–1327 (2005).
Arnalich, F. et al. Relationship between circulating levels of calcitonin gene-related peptide, nitric oxide metabolites and hemodynamic changes in human septic shock. Regul. Pept. 65, 115–121 (1996).
Monneret, G., Pachot, A., Laroche, B., Picollet, J. & Bienvenu, J. Procalcitonin and calcitonin-related peptide decrease LPS-induced TNF production by human circulating blood cells. Cytokine 12, 762–764 (2000).
Cuesta, M. C., Quintero, L., Pons, H. & Suarez-Roca, H. Substance P and calcitonin gene-related peptide increase IL-1β, IL-6 and TNF secretion form human peripheral blood mononuclear cells. Neurochem. Int. 40, 301–306 (2002).
Hernanz, A., Medina, S., de Miguel, E. & Martin-Mola, E. Effect of calcitonin gene-related peptide, neuropeptide Y, substance P, and vasoactive intestinal peptide on interleukin-1β, interleukin-6 and tumor necrosis factor-α production by peripheral whole blood cells from rheumatoid arthritis and osteoarthritis patients. Regul. Pept. 115, 19–24 (2003).
Poyner, D. R. et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 54, 233–246 (2002).
Ono, Y., Okano, I., Kojima, M., Okada, K. & Kangawa, K. Decreased gene expression of adrenomedullin receptor in mouse lungs during sepsis. Biochem. Biophys. Res. Commun. 271, 197–202 (2000).
Spier, A. D. & de Lecea, L. Cortistatin: a member of the somatostatin neuropeptide family with distinct physiological functions. Brain Res. Rev. 33, 228–241 (2000).
Dalm, V. A. Cortistatin rather than somatostatin as a potential endogenous ligand for somatostatin receptors in the human immune system. J. Clin. Endocrinol. Metab. 88, 270–276 (2003). The first study highlighting the possibility that cortistatin, but not somatostatin, is the endogenous immunomodulatory factor of this family of peptides.
Lygren, I., Revhaug, A., Burhol, P. G., Giercksky, K. E. & Jenssen, T. G. Vasoactive intestinal polypeptide and somatostatin in leukocytes. Scand. J. Clin. Lab. Invest. 44, 347–351 (1988).
Krantic, S. Peptides as regulators of the immune system: emphasis on somatostatin. Peptides 21, 1941–1964 (2000).
Gonzalez-Rey, E. et al. Cortistatin, a new anti-inflammatory peptide with therapeutic action in inflammatory bowel disease. Proc. Natl Acad. Sci. USA 103, 4228–4233 (2006).
Gonzalez-Rey, E., Chorny, A., Robledo, G. & Delgado, M. Cortistatin, a new anti-inflammatory peptide with therapeutic action in lethal endotoxemia. J. Exp. Med. 203, 463–471 (2006). References 81 and 82 are the first studies describing the new properties of cortistatin as a potent anti-inflammatory factor.
Deghenghi, R., Papotti, M., Ghigo E. & Muccioli, G. Cortistatin, but not somatostatin, binds to growth hormone secretagogue (GHS) receptors of human pituitary gland. J. Endocrinol. Invest. 24, RC1–RC3 (2001).
Dixit, V. D. et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 114, 57–66 (2004).
Castro, A., Jerez, M. J., Gil, C. & Martinez, A. Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: advances in the development of specific phosphodiesterase inhibitors. Med. Res. Rev. 25, 229–244 (2005).
Yoon, S. W. et al. α-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced tumor necrosis factor-α production in leukocytes by modulating protein kinase A, p38 kinase, and nuclear factor κB signaling pathways. J. Biol. Chem. 278, 32914–32920 (2003). References 86 and 87 provide evidence that cAMP-inducing neuropeptides exert their anti-inflammatory actions by downregulating multiple transduction pathways and transcription factors.
Delgado, M. & Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-κB-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J. Biol. Chem. 276, 369–380 (2001).
Taylor, A. W. The immunomodulating neuropeptide α-melanocyte-stimulating hormone (α-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J. Neuroimmunol. 162, 43–50 (2005).
Chiao, H., Foster, S., Thomas, R., Lipton, J. & Star, R. A. α-Melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J. Clin. Invest. 97, 2038–2044 (1996).
Delgado, M. et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-α and IL-6. J. Immunol. 162, 1200–1205 (1999).
Tuncel, N., Tore, F., Sahinturk, V., Ak, D. & Tuncel, M. Vasoactive intestinal peptide inhibits degranulation and changes granular content of mast cells: a potential therapeutic strategy in controlling septic shock. Peptides 21, 81–89 (2000).
Lam, C. W., Getting, S. J. & Perretti, M. In vitro and in vivo induction of heme oxygenase 1 in mouse macrophages following melanocortin receptor activation. J. Immunol. 174, 2297–2304 (2005).
Delgado, M., Abad, C., Martinez, C., Leceta, J. & Gomariz, R. P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nature Med. 7, 563–568 (2001). The first study showing the therapeutic effect of VIP on an experimental model of inflammatory and autoimmune disease.
Ceriani, G., Diaz, J., Murphree, S., Catania, A. & Lipton, J. M. The neuropeptide α-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation 1, 28–32 (1994).
Gonzalez-Rey, E., Chorny, A., Varela, N., O'Valle, F. & Delgado, M. Therapeutic effect of urocortin on collagen-induced arthritis by downregulating inflammatory and TH1 response and inducing regulatory T cells. Arthritis Rheum. (in the press).
Gonzalez-Rey, E., Chorny, A., O'Valle, F., Varela, N. & Delgado, M. Adrenomedullin protects from experimental arthritis by downregulating inflammation and TH1 responses and inducing regulatory T cells. Am. J. Pathol. (in the press).
Abad, C. et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology 124, 961–971 (2003).
Rajora, N., Boccoli, G., Catania, A. & Lipton, J. M. α-MSH modulates experimental inflammatory bowel disease. Peptides 18, 381–385 (1997).
Gonzalez-Rey, E. et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: downregulation of inflammatory and autoimmune responses. Am. J. Pathol. 168, 1179–1188 (2006).
Keino, H. et al. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch. Ophthalmol. 122, 1179–1184 (2004).
Fernandez-Martin, A., Gonzalez-Rey, E., Chorny, A., Ganea, D. & Delgado, M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 36, 318–326 (2006).
Sato, K., Yamashita, N., Yamashita, N., Baba, M. & Matsuyama, T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity 18, 367–379 (2003). The first study showing that tolerogenic DCs are therapeutically beneficial for the treatment of GVHD in bone-marrow-transplanted animals with leukaemic tumours.
Chorny, A., Gonzalez-Rey, E., Fernandez-Martin, A., Ganea, D. & Delgado, M. Vasoactive intestinal peptide induces regulatory dendritic cells that can prevent acute graft-versus-host disease while maintain graft-versus-tumor. Blood 107, 3787–3794 (2006).
Gatti, S. et al. α-Melanocyte-stimulating hormone protects the allograft in experimental heart transplantation. Transplantation 74, 1678–1684 (2002)
Chorny, A. et al. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc. Natl Acad. Sci. USA 102, 13562–13567 (2005).
Blalock, J. E. The immune system as the sixth sense. J. Intern. Med. 257, 126–138 (2005).
Bangale, Y. et al. VIPase autoantibodies in Fas-defective mice and patients with autoimmune disease. FASEB J. 17, 628–635 (2003).
Herrera, J. L., Fernandez-Montesinos, R., Gonzalez-Rey, E., Delgado, M. & Pozo, D. Protective role for plasmid DNA-mediated VIP gene transfer in non-obese diabetic mice. Ann. NY Acad. Sci. 1070, 337–341 (2006).
Gonzalez-Rey, E., Chorny, A., Del Moral, R. G., Varela, N. & Delgado, M. Therapeutic effect of cortistatin on experimental arthritis by downregulating inflammatory and TH1 responses. Ann. Rheum. Dis. (in the press).
Acknowledgements
We thank the many researchers and laboratories that have contributed to our current understanding of the fundamental parts that neuropeptides play in immunology. This work was supported by grants from the Spanish Ministry of Health, the US National Institutes of Health and the Ramon Areces and La Caixa Foundations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Neurotransmitter
-
A chemical that is used to relay, amplify and modulate electrical signals between a neuron and another cell. Common neurotransmitters include: amino acids, monoamines, acetylcholines, peptides (neuropeptides), purines and nitric oxide.
- Co-stimulatory signal
-
A signal to a T cell (in the form of a soluble or membrane-bound molecule) that has little or no effect on its own, but either enhances or modifies the physiological effect of the primary signal mediated by engagement of the T-cell receptor.
- G-protein-coupled receptor
-
A cell-surface protein of seven membrane-spanning domains that transduces an extracellular signal (ligand binding) into an intracellular cascade through the activation of a guanine nucleotide-binding protein (G protein). The neuropeptide receptors are all members of this family of proteins.
- Microglia
-
Phagocytic cells of myeloid origin that are involved in the innate immune response in the central nervous system. Microglia are considered to be the brain-resident macrophages.
- Chemokines
-
A family of small cytokines that are secreted by different cell types in response to bacterial or viral infections that induce directed chemotaxis in nearby responsive cells. Two major chemokine families are described: CC-chemokines with two adjacent cysteines near the amino terminus, and CXC-chemokines in which cysteines are separated by an amino acid.
- Toll-like receptors
-
(TLRs). A family of receptors that recognize pathogen-associated molecular patterns. TLRs recognize conserved molecular patterns that are common to large groups of microorganisms and/or viruses.
- Delayed-type hypersensitivity
-
A cellular immune response to antigen driven by T helper 1-type cytokines, which induce the infiltration and activation of T cells and macrophages over 24–72 hours.
- Cytotoxic T-lymphocyte-associated protein 4
-
(CTLA4). A T-cell surface protein that, following its ligation to CD80 or CD86 on antigen-presenting cells, negatively signals activated T cells, inducing cell-cycle arrest and inhibiting cytokine production. CTLA4 is constitutively expressed by, and functionally associated with, regulatory T cells.
- Alloreactive
-
The process of responding to antigens that are distinct between members of the same species, such as MHC molecules or blood-group antigens.
- Catecholamine
-
A chemical compound derived from tyrosine that is mainly produced in the adrenal medulla and the postganglionic fibres of the sympathetic nervous system. The most abundant catecholamines are the biogenic amines adrenaline, noradrenaline and dopamine.
- Hypothalamus–pituitary–adrenal axis
-
(HPA axis). A complex set of direct influences and feedback interactions between the paraventricular nucleus of the hypothalamus, which contains neuroendocrine neurons that produce corticotropin-releasing hormone to stimulate the secretion of adrenocorticotropic hormone to the blood in the pituitary gland, which in turn stimulates the production of glucocorticoids in the adrenal cortex. The HPA axis is a major part of the neuroendocrine system, which controls reactions to stress and regulates digestion, energy usage, sexuality and the immune system (having an immunosuppressive action).
- Septic shock
-
A systemic response to severe bacterial infections, which are generally caused by Gram-negative bacterial endotoxins, that leads to a hyperactive and out-of-balance network of inflammatory cytokines, affecting vascular permeability, cardiac function and metabolic balance, and leads to tissue necrosis, multiple-organ failure and death.
- Ghrelin
-
A hormone produced by the stomach that stimulates appetite and the secretion of growth hormone. It is considered to be the counterpart of leptin, which is produced by adipose tissue, and has been associated with anti-inflammatory actions.
- Rheumatoid arthritis
-
An autoimmune disease that leads to chronic inflammation in the joints and subsequent destruction of the cartilage and erosion of the bone. It is divided into two main phases: initiation and establishment of autoimmunity to collagen-rich joint components, and later events associated with the evolving destructive inflammatory processes.
- Crohn's disease
-
A chronic and relapsing inflammatory bowel disease that is characterized by dysfunction of mucosal T cells, altered cytokine production and cellular inflammation that ultimately leads to damage of the distal small intestine and the colonic mucosa, resulting in abdominal pain, rectal bleeding, diarrhoea and weight loss.
- Experimental autoimmune encephalomyelitis
-
(EAE). An inflammatory demyelinating disease of the central nervous system, which shows pathological and clinical similarities to multiple sclerosis. EAE is considered to be an archetypal CD4+ T helper 1 cell (TH1 cell)-mediated autoimmune disease in which TH1 cells that are reactive to components of the myelin sheath infiltrate the nervous parenchyma, release inflammatory cytokines and chemokines, promote leukocyte infiltration and contribute to demyelination.
- Graft-versus-tumour
-
An immune response mounted against tumour cells of an allogeneic bone-marrow transplant by donor T cells (mainly cytotoxic CD8+ T cells) that are derived from the graft.
- Graft-versus-host disease
-
An immune response mounted against the recipient of an allograft (generally in the context of allogeneic bone-marrow transplantation) by donor T cells derived from the graft.
Rights and permissions
About this article
Cite this article
Gonzalez-Rey, E., Chorny, A. & Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol 7, 52–63 (2007). https://doi.org/10.1038/nri1984
Issue Date:
DOI: https://doi.org/10.1038/nri1984
This article is cited by
-
Myopia, corneal endothelial cell density and morphology in a Japanese population-based cross-sectional study: the JPHC-NEXT Eye Study
Scientific Reports (2021)
-
Altered secretory and neuroprotective function of the choroid plexus in progressive multiple sclerosis
Acta Neuropathologica Communications (2020)
-
Vasoactive intestinal peptide dampens formyl-peptide-induced ROS production and inflammation by targeting a MAPK-p47phox phosphorylation pathway in monocytes
Mucosal Immunology (2017)
-
In situ proximity of CX3CR1-positive mononuclear phagocytes and VIP-ergic nerve fibers suggests VIP-ergic immunomodulation in the mouse ileum
Cell and Tissue Research (2017)
-
Linagliptin inhibits lipopolysaccharide-stimulated interleukin-6 production, intranuclear p65 expression, and p38 mitogen-activated protein kinase phosphorylation in human umbilical vein endothelial cells
Renal Replacement Therapy (2016)