Key Points
-
To mediate an effective immune response, lymphocytes must find their way to secondary lymphoid tissues, such as the lymph nodes, the Peyer's patches and the spleen, where antigens and antigen-presenting dendritic cells are selectively localized.
-
When entering lymph nodes and Peyer's patches, lymphocytes selectively adhere to and transmigrate through the high endothelial venules (HEVs), which are distinct from normal venules in several ways.
-
HEVs express unique adhesion molecules (vascular addressins) that function as ligands for lymphocyte homing receptors. They also express several lymphoid chemokines that can activate integrins on circulating lymphocytes.
-
Accumulating evidence indicates that chemokines produced in and around HEVs have a crucial role in lymphocyte trafficking to lymph nodes and Peyer's patches. Nevertheless, the way that chemokines function in vivo is not fully understood.
-
Numerous chemokine-binding molecules are expressed in and immediately around HEVs. Certain chemokines bind preferentially to certain chemokine-binding molecules that seem to be expressed in a concentric manner in the HEV area.
-
We propose that each chemokine-binding molecule provides a substrate to present the appropriate bound chemokine and that lymphocytes expressing appropriate chemokine receptors respond to these matrix-presented chemokines successively. This would result in directed trafficking of lymphocytes across HEVs and into the lymph-node parenchyma.
-
Lymphocytes might respond to these chemokines by haptotaxis and/or chemokinesis, rather than by chemotaxis.
-
Numerous questions remain unanswered concerning the physiology and function of HEVs.
Abstract
Lymphocytes are intrinsically mobile and circulate continuously between the blood and secondary lymphoid tissues. When naive lymphocytes first enter lymph nodes and Peyer's patches, they adhere to and migrate across specific blood vessels known as high endothelial venules (HEVs). The local availability of chemokines in or near HEVs is crucial for the specificity of this process. Here, we summarize recent studies of the chemokine-directed events in lymphocyte trafficking across HEVs, and we examine the dogmas and enigmas concerning lymphocyte migration to lymph nodes and Peyer's patches. A model is also discussed, in which we propose that the response to chemokines immobilized on extracellular-matrix components is important for lymphocyte positioning in vivo.
Similar content being viewed by others
Main
Although the lymphoid system consists of various separate tissues and organs, it functions as a single entity. This is mainly because its principal cellular constituents, lymphocytes, are intrinsically mobile and continuously recirculate in large numbers between the blood and the lymph by way of the secondary lymphoid tissues (which include the lymph nodes, the PEYER'S PATCHES and the spleen), where antigens and antigen-presenting cells are selectively localized1,2,3,4,5. When entering lymph nodes and Peyer's patches, lymphocytes selectively adhere to and transmigrate through HIGH ENDOTHELIAL VENULES (HEVs). Each HEV is composed of a prominent perivascular sheath, a thick BASAL LAMINA and a layer of endothelial cells that have a plump morphology2 (Box 1). They are located mainly in the T-cell zones, such as the paracortical areas of lymph nodes and the interfollicular areas of Peyer's patches, but some are also found in the B-cell zones, particularly in the periphery of B-cell follicles. Anatomically, HEVs are postcapillary venules, but they are distinct from ordinary venules in several aspects, as described in Box 1. In particular, under physiological conditions, HEVs produce certain chemokines constitutively. There is now a growing body of evidence that chemokines produced in and around HEVs have a crucial role in lymphocyte trafficking to lymph nodes and Peyer's patches.
Entry into lymph nodes and Peyer's patches
As illustrated in Fig. 1, lymphocytes and dendritic cells (DCs) enter lymph nodes by two routes6. Most DCs enter lymph nodes in the afferent lymph7,8, although a particular subset of DCs (plasmacytoid DCs) might enter lymph nodes through HEVs9. In the peripheral tissues, most DCs are immature10. These DCs can bind and internalize antigen efficiently but have little ability to present antigen to T cells; however, after encountering antigen, immature DCs are rapidly activated, thereby enabling efficient antigen presentation. Activated DCs are then mobilized from peripheral tissues to lymph nodes. Chemokines, including CC-chemokine ligand 21 (CCL21; also known as secondary lymphoid-tissue chemokine, SLC), have an important role in this migratory process11. Most DCs entering lymph nodes by way of the afferent lymph do not leave in the efferent lymph but settle in the vicinity of HEVs in the paracortex12,13; this seems to occur partly because of the organization of the lymph node and partly because of the local production of chemokines within the lymph node. DCs are probably short-lived, dying in the lymph nodes after they have presented antigen to lymphocytes travelling through the HEV area.
Lymphocytes and dendritic cells (DCs) enter lymph nodes by different routes. Most lymphocytes migrating to lymph nodes enter from the peripheral blood. Although various types of leukocyte are found in the arteries of lymph nodes, only lymphocytes can interact with and extravasate through high endothelial venules (HEVs) to migrate into the lymph-node parenchyma. T and B cells subsequently segregate into the T-cell zones and B-cell zones, respectively. By contrast, most DCs, together with small numbers of lymphocytes, enter lymph nodes through the afferent lymphatics. They then accumulate in the vicinity of HEVs. The HEVs are surrounded by fibroblastic reticular cells (FRCs), which form channels — called FRC conduits — that project from the subcapsular sinus into the T-cell zone. Some chemokines produced extranodally might reach HEVs through the FRC conduit.
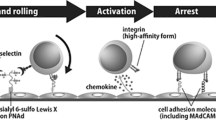
Most lymphocytes enter lymph nodes across HEVs. It has been estimated that one in every four circulating lymphocytes will leave the blood after entering an HEV14. This route of cell migration is highly specific to lymphocytes, and under physiological conditions, HEVs exclude the adhesion and transmigration of other leukocytes (except, probably, plasmacytoid DCs9). Until several years ago, it was thought that such specificity is achieved mainly by the differential expression of adhesion molecules by lymphocytes and by endothelial cells of HEVs, but subsequent studies (discussed later) have emphasized the role of chemokines (Box 2). Numerous reports have confirmed that lymphocytes that migrate preferentially through peripheral lymph nodes express L-selectin, which recognizes peripheral-node addressins (PNADs; highly glycosylated and sulphated sialomucins) on peripheral lymph-node HEVs, whereas lymphocytes that migrate preferentially through the gut express α4β7-integrin, which recognizes mucosal vascular addressin cell-adhesion molecule 1 (MADCAM1) on venules of the intestinal lamina propria and Peyer's patch HEVs2,15. Recent studies indicate that L-selectin ligands (PNADs) on peripheral lymph-node HEVs are synthesized by the coordinated actions of two α1,3-fucosyltransferases (FucT-IV16 and FucT-VII17) and a sulphotransferase known as high endothelial cell N-acetylglucosamine 6-O-sulphotransferase (HEC-GlcNAc6ST18; also known as L-selectin ligand sulphotransferase, LSST19). Engagement of these ligands by L-selectin results in the slowing down and rolling of lymphocytes along HEVs, which brings lymphocytes into close contact with the endothelium, where the endothelial-cell adhesion molecules intracellular adhesion molecule 1 (ICAM1) and ICAM2 are co-expressed. Subsequently, the lymphocytes attach firmly to these adhesion molecules through the cell-surface β2-integrin, lymphocyte function-associated antigen 1 (LFA1). They then transmigrate between adjacent endothelial cells and penetrate the underlying basement membrane to gain access to the parenchyma of the tissue2,15 (Box 2).
Although these processes of firm adhesion to and transmigration through HEVs are unique to lymphocytes, the main 'homing receptor', L-selectin, is shared with other leukocytes, and similarly, one of the main adhesion receptors, LFA1, is expressed by virtually all types of leukocyte. The observation that granulocytes expressing L-selectin roll along but fail to bind to the HEV cell surface20, together with the finding that blocking L-selectin function results in a strong reduction of rolling on HEVs21, indicates that L-selectin functions as a major 'rolling receptor' for leukocytes but that the simultaneous expression of L-selectin and LFA1 by leukocytes does not necessarily result in either firm adhesion to HEVs or migration to the lymph-node parenchyma. In addition, the LFA1 molecules expressed by most leukocytes need to be activated to bind ICAM1 (Ref. 22). Therefore, additional factors are required for rolling lymphocytes to stop and attach firmly to HEVs.
HEV-associated chemokines and cell migration
What provides such an activation stimulus to rolling lymphocytes in HEVs? One hint came from studies using pertussis toxin (an inhibitor of the signalling function of G-protein-coupled receptors, including chemokine receptors), which was shown to strongly inhibit lymphocyte entry into lymph nodes23 and lymphocyte adhesion to HEVs in vivo24. Because both events are dependent on LFA1 (Ref. 25) and chemokines can activate LFA1 (Ref. 26), chemokines have been thought to provide such a stimulus, and chemokine-induced LFA1 activation has been considered to cause the binding of lymphocytes to HEVs. Subsequent studies have indeed confirmed that chemokines that are produced by or are adherent to HEVs provide important cues that control the migration of lymphocytes into and within lymphoid organs27,28.
T-cell migration. CCL21 and CCL19 (also known as Epstein–Barr virus-induced molecule 1 ligand chemokine, ELC) were the first chemokines found to be crucial for lymphocyte migration into lymph nodes (Table 1). The receptor for these chemokines is CC-chemokine receptor 7 (CCR7)29,30, which is expressed by naive lymphocytes and DCs but not by granulocytes or monocytes31. Importantly, CCL21 is produced by the endothelial cells of HEVs32,33 and can induce rapid integrin binding to immobilized ICAM1 (Refs 26,34,35) and MADCAM1 (Ref. 36), under conditions mimicking turbulent blood flow. CCL21 protein is expressed by more than 80% of HEVs in lymph nodes and Peyer's patches37. By contrast, CCL19 is produced by stromal cells in the area surrounding HEVs and seems to be transported to the luminal surface of HEVs38. A mutant mouse strain, plt/plt (plt; paucity of lymph-node T cells), that congenitally lacks the expression of both CCL19 and CCL21 in HEVs33,39,40 shows markedly defective T-cell adhesion to HEVs and therefore defective T-cell migration to lymph nodes41. Subcutaneous injections of CCL19 (Ref. 38) or CCL21 (Ref. 35) can partially restore T-cell trafficking to the draining lymph nodes of plt/plt mice. In addition, mice that are deficient in CCR7 also show severely defective T-cell migration to lymph nodes42. These results indicate that interactions between CCL21/CCL19 and CCR7 are crucial for triggering T-cell adhesion to HEVs, which is obligatory for entry into lymph nodes.
Under in vitro flow conditions, CXC-chemokine ligand 12 (CXCL12; also known as stromal-cell-derived factor 1, SDF1) can rapidly induce T-cell adhesion to ICAM1 by activating LFA1 (Ref. 26), and it can also induce T-cell tethering to vascular cell-adhesion molecule 1 (VCAM1) by clustering the integrin very late antigen 4 (VLA4)43. Although CXCL12 protein is present on the luminal side of HEVs44, mRNA encoding this chemokine cannot be detected in HEVs44; therefore, CXCL12 is probably produced elsewhere and transported to HEVs (as occurs for CCL19). A study by Okada et al.44 indicates that CXCL12, together with its receptor CXC-chemokine receptor 4 (CXCR4), has a secondary role in lymphocyte migration across HEVs. The authors showed that the migration of CXCR4-deficient T cells to lymph nodes and Peyer's patches was substantially impaired when they were transferred to plt/plt mice but not to wild-type mice44. These results indicate that CXCL12 and CXCR4 have a minor role in T-cell migration across HEVs and that CCR7 and its ligands (CCL21 and CCL19) have an important role — although the possibility that CXCL12 has a role in the migration of a small but distinct T-cell subset cannot be excluded. Interestingly, the contribution of CXCR4-mediated signals to lymphocyte migration might differ between mouse strains: T-cell migration to lymph nodes and Peyer's patches was more severely compromised in mice with a plt mutation on the BALB/c background than on the C57BL/6 background44, indicating the existence of strain-specific genetic modifications in some chemokine-signalling pathways45.
CXCL12 and CXCR4 might also be involved in the transmigration process. Studies in rats showed that the transmigration of T cells through cultured HEV cells was strongly inhibited by a CXCR4 antagonist or by the CXCL12-mediated desensitization of T cells46. Furthermore, when CXCL12 was apically expressed on cytokine-stimulated human umbilical vein endothelial cells, it strongly promoted lymphocyte transmigration through the endothelial cells under in vitro flow conditions43,47.
CXCL10 (also known as interferon-inducible protein 10, IP10) is a ligand for CXCR3, which is expressed by interferon-γ-producing CD4+ T cells48 and by plasmacytoid monocytes that are found in and around the HEVs of lymph nodes draining inflamed tissue9. Our analysis49 showed that CXCL10 is also constitutively expressed on a substantial proportion of lymph-node HEVs — although another study50 produced conflicting results. Because mRNA encoding CXCL10 was not detectable in purified HEV cells (using PCR after reverse transcription of RNA) (T.T. and Y. Ebisuno, unpublished observations), but CXCL10 protein was readily detectable49, the protein might be synthesized by non-HEV cells and transported to the HEV area. Although the observation that naive lymphocytes do not express CXCR3 (Ref. 51) virtually excludes a role for CXCL10 in lymphocyte trafficking under physiological conditions, CXCL10 might be involved in the trafficking of activated T cells and plasmacytoid monocytes across HEVs during inflammation.
B-cell migration. Despite both CCL21 (Ref. 52) and CCL19 (Ref. 53) efficiently inducing the chemotaxis of B cells, B-cell migration to lymph nodes is only slightly affected in plt/plt mice41 (which are deficient in these chemokines33,39,40), and desensitization of their receptor, CCR7, failed to affect B-cell adhesion to Peyer's-patch HEVs in vivo54. However, a study by Okada et al.44 indicates that signals through CCR7 do have a role in triggering B-cell adhesion to HEVs, in cooperation with signals mediated by CXCR4. The authors showed that, similar to CXCR4-deficient T cells, intravenously injected CXCR4-deficient B cells migrated to lymph nodes at a low level in the CCR7-ligand-deficient plt/plt mice but not when wild-type mice were used as the recipients, indicating that both CXCR4 and CCR7 contribute to B-cell migration into lymph nodes.
It has been reported that CXCR5 signals have a role in B-cell migration to Peyer's patches but not lymph nodes44. CXCR5 is highly expressed by naive recirculating B cells55. Okada et al.44 reported that CXCL13 (also known as B-lymphocyte chemoattractant, BLC), which is the ligand for CXCR5 (Refs 56,57), is preferentially expressed by HEVs in the follicles of Peyer's patches and that the migration of CXCR5-deficient B cells to Peyer's patches was reduced to 50% of that of wild-type B cells44. However, under their experimental conditions, CXCL13 was undetectable in the HEVs of peripheral lymph nodes, and CXCR5-deficient B cells migrated to these lymph nodes at a level comparable to wild-type B cells. Therefore, they suggested that the chemokine and chemokine receptor requirements for B-cell entry into lymph nodes and Peyer's patches are subtly different44 (Box 3).
By contrast, a separate study by Ebisuno et al.37 contradicts the study by Okada et al.44 in several respects. Ebisuno and colleagues observed that CXCL13 was detectable in approximately 80% of HEVs in lymph nodes and Peyer's patches, as assessed by immunohistochemistry. Luminal expression was observed in approximately 50% of HEVs37. In vivo migration studies showed that fewer B cells migrated to the lymph nodes of CXCL13-deficient mice than to those of wild-type mice, although B cells migrated to the spleen at comparable levels37. These results show that the CXCL13 expressed on the luminal surface of a substantial proportion of HEVs has a crucial role in B-cell trafficking across the HEVs in lymph nodes and Peyer's patches. Similar to CCL19 and CXCL12, CXCL13 is produced mainly by non-HEV cells37 and might be transported to the HEV area through the fibroblastic reticular cell (FRC) network or 'conduit', which is a special delivery system for the transit of soluble factors to HEVs12,58,59 (discussed later).
Migration to the T-cell and B-cell zones
Once inside the lymphoid tissues, naive T cells migrate towards the T-cell zones (interfollicular areas) and naive B cells migrate towards the B-cell zones (follicular areas)60, in response to CCL21/CCL19 and CXCL13, respectively27. A study by Reif et al.61 indicates that the positioning of T and B cells is determined by a balanced responsiveness to CCL21 and CXCL13. For example, naive B cells express high levels of CXCR5 and low levels of CCR7, so they migrate preferentially to follicles where the CXCR5 ligand, CXCL13, is produced. Antigen stimulation then upregulates CCR7 expression, and the altered balance of chemokine responsiveness drives the migration of antigen-stimulated B cells into the T-cell zone.
The sorting of T and B cells into their respective microdomains might also occur at the level of HEVs. Warnock et al.54 observed that, for mouse Peyer's patches, the sites of B- and T-cell firm adhesion in HEVs were geographically segregated: that is, T cells adhered preferentially to INTERFOLLICULAR HEVS that expressed high levels of CCL21, whereas B cells adhered mainly to FOLLICULAR HEVS that expressed only low or undetectable levels of CCL21.
This hypothesis is, however, in contrast to earlier studies of lymph nodes in both mice62 and rats60,63, in which T and B cells were shown to enter lymph nodes through the same HEVs60,62,63. Our previous study37 and ongoing investigations (M.M. and T.T., unpublished observations) indicate that most HEVs express both CCL21 and CXCL13 in the lymph nodes and Peyer's patches; therefore, although T-lymphotropic and B-lymphotropic HEVs might exist, we do not think that they are widespread.
Migration of activated lymphocytes
When tissue DCs presenting antigens from the periphery enter lymphoid tissues, HEVs produce (or bind in some cases) various chemokines that are not present in the HEVs of lymph nodes draining non-inflamed tissue41. Palframan et al.64 reported that CCL2 (also known as monocyte chemotactic protein 1, MCP1) accumulated in the lumen of HEVs in lymph nodes draining inflamed skin, where it triggered rolling monocytes to adhere to these HEVs64. In a similar local inflammation model, Janatpour et al.50 reported that a subset of HEVs in 'inflamed' lymph nodes acquired luminal expression of CXCL9 (also known as monokine induced by interferon-γ, MIG) (probably synthesized by the PARAFOLLICULAR HEV cells) in a tumour-necrosis factor-dependent manner, and these HEVs also preferentially supported monocyte binding and extravasation.
Concurrent with these changes in the chemokine-expression profile of HEVs, the activation of lymphocytes results in marked changes in their chemokine-receptor expression, generating cells with distinct migratory capacities. At an early stage of T-cell activation, CCR7 expression is transiently upregulated, which might help the cells to remain in the T-cell zone for a period of time65,66. Some of the activated CD4+ T cells then rapidly upregulate their expression of CXCR5, while losing CCR7 expression67. These cells migrate to B-cell follicles, where CXCL13 is constitutively expressed, and help antigen-primed B cells to differentiate into antibody-producing cells68,69. Subsequently, a certain fraction of antigen-primed T cells expands clonally and differentiates into central memory and effector memory T cells65,70,71. Central memory T cells express L-selectin and CCR7, so they can migrate to the secondary lymphoid tissues65. When these T cells are restimulated with antigen, they lose CCR7 expression and differentiate into effector memory T cells. Effector memory T cells express low or undetectable levels of L-selectin and CCR7; therefore, they do not usually migrate to lymphoid tissues but rather to the site of infection and inflammation. However, it should be noted that the effector and memory T-cell populations in vivo seem to consist of complex and heterogeneous populations65, and the concept that central memory T cells traffic to lymphoid tissues, whereas effector memory T cells traffic to non-lymphoid tissues, needs to be investigated further.
As well as peptide chemokines, recent studies indicate that lipid chemoattractants, such as leukotriene B4 (LTB4), are also involved in the migration of activated T cells72,73,74. LTB4 is characteristically produced during the degranulation of mast cells. LTB4 is a potent chemoattractant for neutrophils, eosinophils and monocytes; however, it can also attract activated CD4+ and CD8+ T cells that express the high-affinity LTB4 receptor, BLT1. Recently, signalling mediated by the interaction of LTB4 with BLT1 has been shown to induce the firm adhesion of T helper 1 and T helper 2 cells to cultured endothelial cells under flow, as well as the trafficking of effector CD4+ and CD8+ T cells into the airway in an asthma model74. LTB4 has also been shown to induce the chemotaxis of CD8+ T cells in vitro72,73 and the rapid integrin-mediated arrest of rolling CD8+ T cells in the venules of the cremaster muscle72. Therefore, LTB4 might have an important role in the recruitment of both myeloid leukocytes and activated T cells to inflamed sites in vivo. Because mast cells are found not only in peripheral tissues but also in lymph nodes75, their degranulation products might reach HEVs through the FRC conduit in lymph nodes, thereby influencing the trafficking of activated T cells into lymph nodes.
Access of chemokines to HEVs
Studies by Anderson, Gretz, Shaw and others58,59 indicate that the reticular network in the lymph-node cortex functions as a conduit for the delivery of chemokines from the subcapsular sinus to HEVs. After injection of low-molecular-weight fluorescein-labelled molecules into the draining area of a lymph node, not only the subcapsular sinus but also the reticular fibres and the abluminal side of HEVs were immediately visualized59, indicating that lymph-borne molecules move from the subcapsular sinus to the HEVs by way of the reticular network. Interestingly, when high-molecular-weight molecules were injected, they were excluded from the cortical parenchyma, indicating that the conduit functions as a molecular sizing column, allowing only low-molecular-weight molecules to reach the HEVs. The delivery system is termed the FRC conduit because the reticular fibres in the lymph node are ensheathed by FRCs, which thereby creates the conduit space58,59. Gretz et al.58,59 proposed three mechanisms that would allow lymph-borne molecules to enter the FRC conduit and be displayed on the HEV surface: regulated vesicular transport across the cells lining the subcapsular sinus (transcytosis); hydrostatic pressure provided by fluid movement from the capillary near the subcapsular sinus into the conduit; and active transcytotic transport from the abluminal to the luminal surface of HEVs76. Although the exact operating mechanisms are still incompletely characterized, the FRC conduit provides an explanation for the presence of chemokines on the luminal surface of HEVs that do not produce them. A similar FRC conduit system that can transport chemokines was recently found in the spleen77.
Chemokine-binding molecules in HEVs
Although chemokines are instrumental in the movement of lymphocytes into secondary lymphoid tissues, and also in the segregation of microdomains in these tissues, the precise mechanisms by which chemokines work in vivo remain unclear. For example, several chemokines are probably required for the unidirectional movement of a lymphocyte from the inside to the outside of HEVs and for the navigation of a lymphocyte into a certain tissue microdomain, as indicated by the neutrophil studies of Foxman et al.78; however, it is unclear how many chemokines a lymphocyte must encounter and in what order these need to be encountered before a lymphocyte is guided to the right place. Also, it is not known whether lymphocytes in vivo respond to one chemokine at a time or to several chemokines simultaneously. More fundamentally, although the term chemotaxis implies that lymphocytes undergo directed motion in a chemokine gradient, it has not been firmly established whether lymphocytes actually move towards the source of a chemokine in vivo79. In particular, although lymphocyte migration is thought to be governed over long distances by chemotactic gradients, such concentration gradients have not been demonstrated in vivo79. Furthermore, even in vitro, the ability of lymphocytes to undergo directed migration along a chemokine gradient, in the absence of a gravitational force, has not been well documented79,80. We propose that a gradient of soluble chemokines would not remain stable under the in vivo conditions of high blood flow at the surface of the endothelium, although the alternative possibility is that some net flow may be necessary to prevent the build-up of high concentrations of chemokines, which would abolish a meaningful gradient. Indeed, in vitro studies indicate that transendothelial migration of lymphocytes can occur in the absence of a gradient, provided that a high concentration of chemokines is immobilized on the luminal surface and that continuous physiological shear stress is applied on the migrating lymphocytes43,46.
Traditionally, glycosaminoglycans and proteoglycans were thought to bind and present chemokines81. We have shown that heparan sulphate proteoglycans are expressed by HEVs (T.T., unpublished observations); however, neither the molecular nature of the core protein nor its ability to present chemokines to lymphocytes in HEVs is known. At present, there is no information regarding the expression of chondroitin sulphate proteoglycans by HEVs. Although we did not observe positive staining of HEVs using a monoclonal antibody specific for chondroitin sulphate (CS56) (T.T., unpublished observations), this antibody does not recognize highly sulphated chondroitin sulphates, such as chondroitin sulphates B and E, which have been shown to bind certain lymphoid chemokines, including CCL21 (Refs 82,83).
During our attempts to identify genes expressed specifically by HEVs, we found divergent classes of molecules that can bind humoral factors, including chemokines, within or in the vicinity of HEVs. One of these molecules, endoglin (CD105)84, is a co-receptor for transforming growth factor-β (TGF-β)85. Another TGF-β-binding protein expressed by HEVs is the leucine-rich HEV glycoprotein (LRHG), the expression of which is restricted to HEV cells86. LRHG is a 342-amino-acid secretory protein containing 8 tandem leucine-rich repeats of 24 amino acids each, which has a high degree of homology to human leucine-rich α2-glycoprotein. TGF-β inhibits lymphocyte adhesion to cytokine-activated endothelial cells87. Although little is known about the expression of TGF-β in the lymph-node paracortex (including in HEVs), it is tempting to speculate that endoglin and/or LRHG localized to the HEV area serve as anchoring molecules for TGF-β, thereby helping the cytokine to be immobilized in the vicinity of HEVs and to regulate lymphocyte adhesiveness and migration in this area.
In the lumen of HEVs, an interesting chemokine-binding molecule, DARC (Duffy antigen receptor for chemokines), is abundantly expressed88,89. DARC, similar to the chemokine receptors, belongs to the family of seven-transmembrane-spanning molecules, but it lacks the DRY MOTIF that is present in the cytoplasmic domain of chemokine receptors and is required for G-protein coupling90. Therefore, DARC should be unable to transmit chemokine signals intracellularly, so it might function as a scavenger receptor91. Indeed, functional studies indicate that DARC can downregulate the activities of pro-inflammatory chemokines by binding and sequestering them89. Furthermore, HEVs constitutively express the pro-inflammatory chemokine CXCL1 (KC) at both the mRNA and protein levels (T.T. and Y. Ebisuno, unpublished observations), but HEVs do not usually allow the trafficking of neutrophils and monocytes, which express receptors for CXCL1. It is therefore tempting to speculate that DARC masks or nullifies the functional activity of CXCL1 on the surface of HEVs; however, because DARC-deficient mice show no abnormality in the cellular composition of their lymph nodes89, DARC seems to be dispensable for the control of lymphocyte trafficking to lymph nodes and might be functionally substituted by other molecule(s). Currently, the functional significance of CXCL1 expression by HEVs remains unclear.
In the basal lamina of HEVs, another protein that can bind chemokines is expressed — MAC25. It is a secreted protein, with a molecular mass of approximately 30 kDa, which was previously identified as insulin-like growth factor-binding protein related protein 1 (IGFBPrP1)92 and tumour-derived adhesion factor93 (which was later renamed angiomodulin94). Structurally, MAC25 has a cysteine-rich domain containing the IGFBP motif and an immunoglobulin-like domain95. It is expressed in the basal lamina of small blood vessels (including HEVs) in lymph nodes and Peyer's patches but not in other tissues49,96. Although Girard et al.88 have reported that MAC25 is expressed by the luminal and basolateral faces of HEVs, our study96 indicates that its expression is limited to the basal lamina. As previously speculated88, MAC25 can bind certain humoral factors, such as vascular endothelial growth factor96, as well as certain chemokines, including CXCL10, CCL5 (also known as regulated upon activation, normal T-cell expressed and secreted, RANTES) and CCL21 (Ref. 49), but it does not bind any of the pro-inflammatory chemokines tested49. Interestingly, MAC25 co-localizes with CXCL10 and CCL21 in the basal lamina of HEVs49. Furthermore, it can present these chemokines to receptor-expressing cells in vitro49. These results indicate that MAC25 might present certain chemokines in situ on the basal lamina of HEVs, although further studies are required to determine the exact localization of MAC25.
Some extracellular-matrix proteins that are present in the basal lamina of HEVs can also bind certain chemokines. For example, collagen IV binds CCL21 and CXCL10 (Ref. 49), and fibronectin binds CXCL12 (Ref. 97). Laminin also binds certain chemokines preferentially (T.T. and B. Yang, unpublished observations).
How do HEV-associated chemokines function?
So, several chemokine-binding molecules are expressed by HEVs and their local environment. In three dimensions, we propose that these chemokine-binding molecules form a set of nested cylinders around HEVs that would appear as a set of concentric rings in cross-section, as shown in Fig. 2. If chemokines are differentially displayed on these concentric rings, extravasating lymphocytes that express the appropriate chemokine receptors would recognize these chemokines sequentially, thereby responding to them one after the other. According to this hypothesis, concentration gradients of soluble chemokines are not necessarily required; instead, chemokines deposited on extracellular matrix components have an important role in guiding extravasating lymphocytes to the appropriate domains. Although not verified experimentally, matrix-bound chemokines might function with or without forming a gradient (Fig. 3).
We propose that several chemokine-binding molecules are expressed in a concentric manner in HEVs and their surrounds. The coordinated actions of chemokines and chemokine-binding molecules in and around HEVs are shown schematically in sequential order. a | In the lumen of HEVs, heparan sulphate proteoglycans (red) can capture and present a lymphoid chemokine (X, green) in situ. Duffy antigen receptor for chemokines (DARC; black) — a non-signalling chemokine receptor — captures and scavenges inflammatory chemokines, such as CXC-chemokine ligand 1 (CXCL1; pink), which is constitutively produced by HEVs. b | In the basal lamina of HEVs, another chemokine-binding protein MAC25 (dark blue) can capture a chemokine, such as CC-chemokine ligand 21 (CCL21), CXCL10 or CCL5 (Y, light blue). In addition, other components in the basal lamina such as collagen and fibronectin (grey) can capture a different chemokine (Z, yellow) expressed in the HEV area. c | When lymphocytes rolling along the HEV endothelium recognize chemokine X presented in the lumen, they are signalled to activate their integrins and firmly adhere to HEVs. d | Subsequently, some of the adhering lymphocytes migrate across HEVs and are stimulated by chemokine Y, which is immobilized on MAC25. e | Farther outside, other chemokine-binding molecules, such as collagen IV and fibronectin, can capture a different chemokine (Z). Some of the extravasating lymphocytes might interact with chemokine Z and be stimulated to move into this area. f | By sequentially recognizing the multiple chemokines presented on the tissue matrix, more and more lymphocytes extravasate and progressively move from the inside to the outside of HEVs.
Concentration gradients of soluble chemokines might not be required to account for the directed movement of lymphocytes; instead, chemokines deposited on extracellular-matrix components could be important in guiding extravasating lymphocytes to the appropriate domains. Matrix-bound chemokines (X, Y, Z) can function with or without forming a gradient. a | In the presence of such a chemokine gradient, lymphocytes respond by haptotaxis, which is defined as migration along a gradient of molecules that are bound to the extracellular matrix. b | In the absence of such a gradient, as long as the matrix-presented chemokines are located close enough to each other in multiple rings, lymphocytes can still respond by chemokinesis, which is defined as a chemoattractant-induced increased mobility of cells with random, non-directed movement. Receptor desensitization would inhibit the cells from responding to the chemokines that stimulated them previously, thereby preventing the cells from returning to their original location and helping them move directionally from the inside to the outside of the high endothelial venules.
These hypothetical considerations of chemokine presentation on membrane-bound proteins, however, are not readily reconciled with recent studies that use INTRAVITAL MULTI-PHOTON LASER MICROSCOPY to observe lymph nodes98,99. Cahalan's group98,99 reported that in mouse inguinal lymph nodes, CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester)-labelled T cells obtained from T-cell receptor transgenic mice displayed robust motility, with each cell taking an independent and seemingly random three-dimensional path through the T-cell areas of the lymph node. It could be that lymphocytes stimulated by a luminally presented chemokine(s) acquire robust motility and 'wander' around the lymph-node parenchyma until they encounter the next chemokine. From there, they might be directed to the appropriate microdomains by matrix-presented chemokines. Nonetheless, it remains to be seen whether these observations can be extrapolated to describe the behaviour of lymphocytes in lymph nodes present at different locations, or to describe lymphocytes with a different antigen specificity, or — perhaps the most difficult task — to describe the lymphocytes of different species. Further investigation will be required to obtain more insight into this issue.
Future directions
Although there is compelling evidence that chemokines can induce directed movement of cells in vitro (chemotaxis), there has been little demonstration in vivo that lymphocytes can actually undergo directed motion in response to a chemokine gradient. In the lumen of HEVs and also in the lymph-node parenchyma, the presence of blood or lymph flow might prevent soluble chemokines from forming stable concentration gradients. We propose that unless chemokines are displayed locally in a regulated manner, chemokine-receptor-expressing lymphocytes would not be able to sense these chemokines and thereby would fail to migrate into appropriate sites. We also suggest that lymphocytes might respond to chemokines, not necessarily by chemotaxis, but by haptotaxis and/or chemokinesis (Fig. 3). Although this hypothesis is based on inference and needs to be experimentally verified, it could be the basis of a better understanding of how chemokines function locally to regulate lymphocyte trafficking.

Apart from this issue, there are several other unanswered questions regarding the physiological function of HEVs. For example, what are the signals that regulate the opening and closing of HEV junctions during the course of lymphocyte transmigration across HEVs? HEVs express numerous junctional adhesion molecules that are involved in homophilic cell adhesion, such as JAMs, claudins and occludins; these seem to become disengaged in a coordinated manner subsequent to the adhesion of lymphocytes to endothelial cells. The molecular details of this 'open sesame' signal for the HEV endothelial cells remain uncharacterized. HEVs might also function as sites for the regulation of innate immunity, because the endothelial cells themselves express certain Toll-like receptors (TLRs) (T.T., unpublished observations) and respond to bacterial-cell components100. DCs expressing various TLRs are also preferentially localized in the same vicinity. Although certain chemokines might use the FRC conduits to gain access to HEVs, it remains unclear how certain DC subsets (such as those trafficking across HEVs and those derived from afferent lymphatics) accumulate in this area. We also do not know what induces and maintains the unique phenotype of HEVs in vivo. Although we know that endothelial cells derived from HEVs rapidly lose their phenotype after in vitro culture, we do not know how to prevent this from occurring — and the in vivo control mechanisms of the HEV phenotype are probably more complex. Furthermore, molecular mechanisms underlying the specialization to a 'peripheral' or 'mucosal' HEV phenotype15 are poorly understood. Finally, a master control gene for the growth and differentiation of HEVs remains to be identified. (In this regard, a recently identified nuclear factor, NF-HEV, is interesting, because it is preferentially expressed by HEVs101.) Identification and characterization of such a gene would have considerable clinical applications: it would enable the design of protocols to induce the formation of HEVs in various tissues, including tumours, improving vaccination strategies against pathogens and cancers. It would also help the design of effective protocols for inhibiting the formation of HEVs or similar structures that mediate sustained lymphocyte recruitment to sites of allergic or autoimmune inflammation. Therefore, HEVs will continue to attract our attention by offering a treasury of as-yet-unknown genes and molecules with potential clinical importance.
References
Butcher, E. C. The regulation of lymphocyte traffic. Curr. Top. Microbiol. Immunol. 128, 85–122 (1986).
Kraal, G. & Mebius, R. E. High endothelial venules: lymphocyte traffic control and controlled traffic. Adv. Immunol. 65, 347–395 (1997).
Gowans, J. L. in Adhesion Molecules and Chemokines in Lymphocyte Trafficking (ed. Hamann, A.) ix–xi (Harwood Academic Publishers, Amsterdam, 1997).
Westermann, J. & Pabst, R. How organ-specific is the migration of 'naïve' and 'memory' T cells? Immunol. Today 17, 278–282 (1996).
Pabst, R. & Westermann, J. in Adhesion Molecules and Chemokines in Lymphocyte Trafficking (ed. Hamann, A.) 21–37 (Harwood Academic Publishers, Amsterdam, 1997).
Yoffey, J. M. & Courtice, F. C. in Lymphatics, Lymph and the Lymphomyeloid Complex (eds Yoffey, J. M. & Courtice, F. C.) 539–551 (Academic Press, London, 1970).
Smith, J. B., McIntosh, G. H. & Morris, B. The traffic of cells through tissues: a study of peripheral lymph in sheep. J. Anat. 107, 87–100 (1970).
Hein, W. R., McClure, S. J. & Miyasaka, M. Cellular composition of peripheral lymph and skin of sheep defined by monoclonal antibodies. Int. Arch. Allergy Appl. Immunol. 84, 241–246 (1987).
Cella, M. et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nature Med. 5, 919–923 (1999).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Cavanagh, L. L. & von Andrian, U. Travellers in many guises: the origins and destinations of dendritic cells. Immunol. Cell Biol. 80, 448–462 (2002).
Gretz, J. E., Anderson, A. O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997). This paper, together with references 58 and 59, describes a special system for delivering small molecules from the subcapsular sinus to the HEVs in lymph nodes.
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Hay, J. B. & Hobbs, B. B. The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J. Exp. Med. 145, 31–44 (1977).
Butcher, E. C. et al. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72, 209–253 (1999).
Homeister, J. W. et al. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 (2001).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Hemmerich, S. et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 15, 237–247 (2001).
Hiraoka, N. et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewisx, an L-selectin ligand displayed by CD34. Immunity 11, 79–89 (1999).
Warnock, R. A. et al. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 187, 205–216 (1998).
von Andrian, U. H. & M'Rini, C. In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes. Commun. 6, 85–96 (1998).
Dustin, M. L. & Springer, T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341, 619–624 (1989).
Spangrude, G. J., Baaten, B. A. & Daynes, R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J. Immunol. 132, 354–362 (1984).
Bargatze, R. F. & Butcher, E. C. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J. Exp. Med. 178, 367–372 (1993).
Hamann, A. et al. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J. Immunol. 140, 693–699 (1988).
Campbell, J. J. et al. Chemokines and the arrest of lymphocyte rolling under flow conditions. Science 279, 381–384 (1998). The first evidence that chemokines can induce arrest of rolling lymphocytes under flow conditions.
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
Kunkel, E. J. & Butcher, E. C. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002).
Campbell, J. J. et al. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3-β receptor CCR7. J. Cell Biol. 141, 1053–1059 (1998).
Yoshida, R. et al. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem. 273, 7118–7122 (1998).
Nagira, M. et al. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J. Biol. Chem. 272, 19518–19524 (1997).
Gunn, M. D. et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naïve T lymphocytes. Proc. Natl Acad. Sci. USA 95, 258–263 (1998).
Vassileva, G. et al. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 190, 1183–1188 (1999).
Tangemann, K., Gunn, M. D., Giblin, P. & Rosen, S. D. A high-endothelial cell derived chemokine induces rapid, efficient, and subset-specific arrest of rolling T lymphocytes on a reconstituted endothelial substrate. J. Immunol. 161, 6330–6337 (1998).
Stein, J. V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Pachynski, R. K., Wu, S. W., Gunn, M. D. & Erle, D. J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin α4β7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 161, 952–956 (1998).
Ebisuno, Y. et al. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J. Immunol. 171, 1642–1646 (2003). The first paper to describe that the expression of CXCL13 in the HEV lumen is important for B-cell trafficking across HEVs in both lymph nodes and Peyer's patches.
Baekkevold, E. S. et al. The CCR7 ligand ELC (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193, 1105–1111 (2001).
Luther, S. A. et al. Coexpression of the chemokine ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl Acad. Sci. USA 97, 12694–12699 (2000).
Nakano, H. & Gunn, M. D. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 166, 361–369 (2001).
Nakano, H. et al. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 27, 215–221 (1997).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Grabovsky, V. et al. Subsecond induction of α4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J. Exp. Med. 192, 495–506 (2000).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002). This study delineates the importance of CCR7 and CXCR4 in transmitting integrin-activating signals to rolling T and B cells in HEVs.
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol. 3, 867–878 (2003).
Phillips, R. & Ager, A. Activation of pertussis toxin-sensitive CXCL12 (SDF-1) receptors mediates transendothelial migration of T lymphocytes across lymph node high endothelial cells. Eur. J. Immunol. 32, 837–847 (2002).
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nature Immunol. 2, 515–522 (2001).
Yamamoto, J. et al. Differential expression of the chemokine receptors by the TH1- and TH2-type effector populations within circulating CD4+ T cells. J. Leukoc. Biol. 68, 568–574 (2000).
Nagakubo, D. et al. A high endothelial venule secretory protein, mac25/angiomodulin, interacts with multiple high endothelial venule-associated molecules including chemokines. J. Immunol. 171, 553–561 (2003). The first study to show that a component of the basal lamina of HEVs can bind lymphoid chemokines preferentially and present them to receptor-expressing cells.
Janatpour, M. J. et al. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 194, 1375–1384 (2001).
Loetscher, M. et al. Chemokine receptor specific for IP10 and MIG: structure, function, and expression in activated T lymphocytes. J. Exp. Med. 184, 963–969 (1996).
Yoshida, R. et al. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int. Immunol. 10, 901–910 (1998).
Nagira, M. et al. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 28, 1516–1523 (1998).
Warnock, R. A. et al. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000). The authors use intravital microscopy to show the presence of T- and B-lymphotropic HEVs. This paper also shows that CCL21 expression by HEVs closely correlates with preferential T-cell adhesion to HEVs.
Forster, R., Emrich, T., Kremmer, E. & Lipp, M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84, 830–840 (1994).
Gunn, M. D. et al. A B-cell homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Legler, D. F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Gretz, J. E., Kaldjian, E., Anderson, A. O. & Shaw, S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J. Immunol. 157, 495–499 (1996).
Gretz, J. E. et al. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Ford, W. L. Lymphocyte migration and immune responses. Prog. Allergy 19, 1–59 (1975).
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416, 94–99 (2002).
Gutman, G. A. & Weissman, I. L. Homing properties of thymus-independent follicular lymphocytes. Transplantation 16, 621–629 (1973).
Nieuwenhuis, P. & Ford, W. L. Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell. Immunol. 23, 254–267 (1976).
Palframan, R. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Muller, G. & Lipp, M. Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Microcirculation 10, 325–334 (2003).
Willimann, K. et al. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 28, 2025–2034 (1998).
Ansel, K. M. et al. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Sallusto, F., Palermo, B., Hoy, A. & Lanzavecchia, A. The role of chemokine receptors in directing traffic of naive, type 1 and type 2 T cells. Curr. Top. Microbiol. Immunol. 246, 123–128 (1999).
Sallusto, F., Mackay, C. R. & Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18, 593–620 (2000).
Goodarzi, K. et al. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nature Immunol. 4, 965–973 (2003).
Ott, V. L. et al. Mast-cell dependent migration of effector CD8+ T cells through production of leukotriene B4 . Nature Immunol. 4, 974–981 (2003).
Tager, A. M. et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nature Immunol. 4, 982–990 (2003).
Bessis, M. in Living Blood Cells and Their Ultrastructure 543 (Springer, Berlin, 1973).
Middleton, J. et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–395 (1997).
Nolte, M. A. et al. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 198, 505–512 (2003).
Foxman, E. F., Campbell, J. J. & Butcher, E. C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139, 1349–1360 (1997).
Wei, S. H., Parker, I., Miller, M. J. & Cahalan, M. D. A stochastic view of lymphocyte motility and trafficking within the lymph node. Immunol. Rev. 195, 136–159 (2003). This paper indicates that lymphocytes might respond to matrix-immobilized chemokines.
Wilkinson, P. C. Assays of leukocyte locomotion and chemotaxis. J. Immunol. Methods 216, 139–153 (1998).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Hirose, J. et al. Versican interacts with chemokines and modulates cellular responses. J. Biol. Chem. 276, 5228–5234 (2001).
Kawashima, H. et al. Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/IdoAα1-3GalNAc(4,6-O-disulfate) interacts with L- and P-selectin and chemokines. J. Biol. Chem. 277, 12921–12930 (2002).
Izawa, D. et al. Expression profile of active genes in mouse lymph node high endothelial cells. Int. Immunol. 11, 1989–1998 (1999).
Cheifetz, S. et al. Endoglin is a component of the transforming growth factor-β receptor system in human endothelial cells. J. Biol. Chem. 267, 19027–19030 (1992).
Saito, K. et al. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. J. Immunol. 168, 1050–1059 (2002).
Chin, Y. -H., Ye, M. -W., Cai, J. -P. & Wu, X. -M. Differential regulation of tissue-specific lymph node high endothelial venule cell adhesion molecules by tumor necrosis factor and transforming growth factor-β1. Immunology 87, 559–565 (1996).
Girard, J. -P. et al. Heterogeneity of endothelial cells. The specialized phenotype of human high endothelial venules characterized by suppression subtractive hybridization. Am. J. Pathol. 155, 2043–2055 (1999).
Kashiwazaki, M. et al. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int. Immunol. 15, 1219–1227 (2003).
Neote, K., Mak, J. Y., Kolakowski, L. F. Jr & Schall, T. J. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood 84, 44–52 (1994).
Hadley, T. J. & Peiper, S. C. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood 89, 3077–3091 (1997).
Baxter, R. C. et al. Recommendations for nomenclature of the insulin-like growth factor binding protein superfamily. Endocrinology 139, 4036 (1998).
Akaogi, K. et al. Specific accumulation of tumor-derived adhesion factor in tumor blood vessels and in capillary tube-like structures of cultured vascular endothelial cells. Proc. Natl Acad. Sci. USA 93, 8384–8389 (1996).
Sato, J. et al. Identification of cell-binding site of angiomodulin (AGM/TAF/Mac25) that interacts with heparan sulfates on cell surface. J. Cell. Biochem. 75, 187–195 (1999).
Hwa, V., Oh, Y. & Rosenfeld, R. G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 20, 761–787 (1999).
Usui, T. et al. Characterization of mac25/angiomodulin expression by high endothelial venule cells in lymphoid tissues and its identification as an inducible marker for activated endothelial cells. Int. Immunol. 14, 1273–1282 (2002).
Pelletier, A. J. et al. Presentation of chemokine SDF-1α by fibronectin mediates directed migration of T cells. Blood 96, 2682–2690 (2000).
Miller, M. J., Wei, S. H., Cahalan, M. D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl Acad. Sci. USA 100, 2604–2609 (2003). Together with reference 99, this paper shows that, after extravasation through HEVs, T cells move rapidly in the lymph-node parenchyma in a seemingly random manner.
Miller, M. J. et al. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl Acad. Sci. USA 101, 998–1003 (2004).
Twisk, A. J., Groeneveld, P. H. & Kraal, G. The effects of bacterial lipopolysaccharide (LPS) on high endothelial venules and interdigitating cells in mouse lymph nodes. Immunobiology 176, 410–422 (1988).
Baekkevold, E. S. et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79 (2003).
Streeter, P. R., Rouse, B. T. & Butcher, E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Cahill, R. N. P., Poskitt, D., Frost, H. & Trnka, Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J. Exp. Med. 145, 420–428 (1977).
Streeter, P. R. et al. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331, 41–46 (1988).
Nakache, M., Berg, E. L., Streeter, P. R. & Butcher, E. C. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature 337, 179–181 (1989).
Stevens, S. K., Weissman, I. L. & Butcher, E. C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J. Immunol. 128, 844–851 (1982).
Sprent, J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell. Immunol. 7, 10–39 (1973).
Westermann, J. et al. IFN-γ influences the migration of thoracic duct B and T lymphocyte subsets in vivo. Random increase in disappearance from the blood and differential decrease in reappearance in the lymph. J. Immunol. 150, 3843–3852 (1993).
Blaschke, V., Micheel, B., Pabst, R. & Westermann, J. Lymphocyte traffic through lymph nodes and Peyer's patches of the rat: B- and T-cell-specific migration patterns within the tissue, and their dependence on splenic tissue. Cell Tissue Res. 282, 377–386 (1995).
Kim, C. H. et al. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108, 1331–1339 (2001).
Debes, G. F., Hopken, U. E. & Hamann, A. In vivo differentiated cytokine-producing CD4+ T cells express functional CCR7. J. Immunol. 168, 5441–5447 (2002).
Acknowledgements
We thank R. Pabst, J. Westermann and T. Hirata for critically reading the manuscript. We also thank members of the Laboratory of Molecular and Cellular Recognition for discussions and suggestions. We thank the Japanese Ministry of Education, Culture, Sports, Science and Technology for continuous grant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- PEYER'S PATCHES
-
Groups of lymphoid nodules that are present in the small intestine (usually the ileum). They occur massed together on the intestinal wall, opposite the line of attachment of the mesentery. Peyer's patches consist of a dome area, B-cell follicles and interfollicular T-cell areas. High endothelial venules are present mainly in the interfollicular areas.
- HIGH ENDOTHELIAL VENULES
-
(HEVs). Venules (small veins that join capillaries to larger veins) that have a high-walled endothelium and are present in the paracortex of lymph nodes and tonsils, as well as in the interfollicular areas of Peyer's patches.
- BASAL LAMINA
-
A supporting structure located at the boundary between endothelia (or epithelia) and the underlying connective tissue. In blood vessels, the basal lamina surrounds the endothelial cells and pericytes, providing physical support. It is composed mainly of collagen IV and laminin molecules. Fibronectin is also present on the connective-tissue face. The basal lamina also seems to be involved in maintaining and modulating endothelial-cell functions by capturing various humoral factors.
- INTERFOLLICULAR HEVS
-
High endothelial venules (HEVs) located in the interfollicular area — the T-cell-dependent area of the lymph-node cortex, which lies between B-cell follicles. Most HEVs belong to this category.
- FOLLICULAR HEVS
-
High endothelial venules (HEVs) located within B-cell follicles.
- PARAFOLLICULAR HEVS
-
High endothelial venules (HEVs) found near, but not within, B-cell follicles.
- DRY MOTIF
-
An amino-acid motif composed of aspartic acid (D), arginine (R) and tyrosine (Y). It is highly conserved among G-protein-coupled receptors and is thought to be essential for G-protein-mediated signalling.
- INTRAVITAL MULTI-PHOTON LASER MICROSCOPY
-
Multi-photon laser microscopy combines the advanced optical techniques of laser-scanning confocal microscopy with long-wavelength multi-photon fluorescence excitation to capture high-resolution, three-dimensional images of living cells and/or tissues that have been labelled with fluorophores. It provides a greater tissue imaging depth (up to 350 μm depending on the tissue) and less photobleaching and phototoxicity than conventional imaging methods.
Rights and permissions
About this article
Cite this article
Miyasaka, M., Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4, 360–370 (2004). https://doi.org/10.1038/nri1354
Issue Date:
DOI: https://doi.org/10.1038/nri1354
This article is cited by
-
Reprogramming of sentinel lymph node microenvironment during tumor metastasis
Journal of Biomedical Science (2022)
-
The role of lymphatics in intestinal inflammation
Inflammation and Regeneration (2021)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
Robo4 contributes to the turnover of Peyer's patch B cells
Mucosal Immunology (2020)
-
Prolonged residence of an albumin–IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis
Nature Biomedical Engineering (2020)