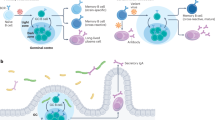
Abstract
The immune system has a memory that it exhibits in the enhanced and augmented responses the second time it meets an antigen. The memory is the result of a number of changes to the system brought about during the primary response. The most important of these changes is the formation of an expanded pool of antigen-specific memory cells. One of the enduring questions in immunology is how these memory cells are maintained for such long periods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gray, D. & Skarvall, H. B cell memory is short-lived in the absence of antigen. Nature 336, 70–73 (1988).
Gray, D. & Matzinger, P. T cell memory is short-lived in the absence of antigen. J. Exp. Med. 174, 969–974 (1991).
Oehen, S., Waldner, H., Kündig, T. M., Hengartner, H. & Zinkernagel, R. M. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J. Exp. Med. 176, 1273–1281 (1992).
Tew, J. G. & Mandel, T. E. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology 37, 69–76 (1979).
Fu, Y. X., Huang, G., Wang, Y. & Chaplin, D. D. Lymphotoxin-α-dependent spleen microenvironment supports the generation of memory B cells and is required for their subsequent antigen-induced activation. J. Immunol. 164, 2508–2514 (2000).
Mims, C. A., Nash, A. & Stephen, J. Pathogenesis of Infectious Disease (Academic Press, London, 2000).
Ciurea, A. et al. Persistence of lymphocytic choriomeningitis virus at very low levels in immune mice. Proc. Natl Acad. Sci. USA 96, 11964–11969 (1999).
Hamad, A. A. et al. Chronic Plasmodium falciparum infections under low intensity malaria transmission in the Sudan. Parasitology 120, 447–456 (2000).
Marrack, P. et al. Homeostasis of αβ TCR+ T cells. Nature Immunol. 1, 107–111 (2000).
Freitas, A. A. & Rocha, B. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 18, 83–111 (2000).
Selin, L. K. et al. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11, 733–742 (1999).
Tough, D. F., Borrow, P. & Sprent, J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272, 1947–1950 (1996).
Zhang, X., Sun, S., Hwang, I., Tough, D. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Mattei, F., Schiavoni, G., Belardelli, F. & Tough, D. F. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167, 1179–1187 (2001).
Lodolce, J. P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Mims, C. A. Pathogenesis of Infectious Disease 3rd edn (Academic Press, London, 1987)
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Lauvau, G. et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294, 1735–1739 (2001).
Sercarz, E. E. et al. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11, 729–766 (1993).
Chen, W., Anton, L. C., Bennink, J. R. & Yewdell, J. W. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12, 83–93 (2000).
Moskophidis, D., Lechner, F., Pircher, H. & Zinkernagel, R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187, 1383–1393 (1998).
Swain, S. L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999).
Tanchot, C., Lemonnier, F. A., Perarnau, B., Freitas, A. A. & Rocha, B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 276, 2057–2062 (1997).
Raff, M. C. Social controls on cell survival and cell death. Nature 356, 397–400 (1992).
McLean, A. R., Rosado, M. M., Agenes, F., Vasconcellos, R. & Freitas, A. A. Resource competition as a mechanism for B cell homeostasis. Proc. Natl Acad. Sci. USA 94, 5792–5797 (1997).
Lau, L. L., Jamieson, B. D., Somasundaram, T. & Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 369, 648–652 (1994).
Hou, S., Hyland, L., Ryan, K. W., Portner, A. & Doherty, P. C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369, 652–654 (1994).
Bruno, L., Kirberg, J. & von Boehmer, H. On the cellular basis of immunological T cell memory. Immunity 2, 37–43 (1995).
Markiewicz, M. A. et al. Long-term T cell memory requires the surface expression of self- peptide/major histocompatibility complex molecules. Proc. Natl Acad. Sci. USA 95, 3065–3070 (1998).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 (1999).
Garcia, S., DiSanto, J. & Stockinger, B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity 11, 163–171 (1999).
Ernst, B., Lee, D. S., Chang, J. M., Sprent, J. & Surh, C. D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Tan, J. T. et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl Acad. Sci. USA 98, 8732–8737 (2001).
van Essen, D., Dullforce, P., Brocker, T. & Gray, D. Cellular interactions involved in TH memory. J. Immunol. 165, 3640–3646 (2000).
Colle, J. H., Truffa-Bachi, P. & Freitas, A. A. Secondary antibody responses to thymus-independent antigens. Decline and life-span of memory. Eur. J. Immunol. 18, 1307–1314 (1988).
Maruyama, M., Lam, K. P. & Rajewsky, K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407, 636–642 (2000).
Gu, H., Tarlinton, D., Muller, W., Rajewsky, K. & Forster, I. Most peripheral B cells in mice are ligand selected. J. Exp. Med. 173, 1357–1371 (1991).
Viale, A. C., Coutinho, A., Heyman, R. A. & Freitas, A. A. V region dependent selection of persistent resting peripheral B cells in normal mice. Int. Immunol. 5, 599–605 (1993).
Rosado, M. M. & Freitas, A. A. The role of the B cell receptor V region in peripheral B cell survival. Eur. J. Immunol. 28, 2685–2693 (1998).
Zinkernagel, R. M. et al. On immunological memory. Annu. Rev. Immunol. 14, 333–367 (1996).
Kundig, T. M. et al. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl Acad. Sci. USA 93, 9716–9723 (1996).
Ochsenbein, A. F. et al. Protective long-term antibody memory by antigen-driven and T helper-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl Acad. Sci. USA 97, 13263–13268 (2000).
Ochsenbein, A. F. et al. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc. Natl Acad. Sci. USA 96, 9293–9298 (1999).
Schittek, B. & Rajewsky, K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature 346, 749–751 (1990).
Altman, J. D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Manz, R. A., Lohning, M., Cassese, G., Thiel, A. & Radbruch, A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10, 1703–1711 (1998).
Jacob, J. & Baltimore, D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature 399, 593–597 (1999).
Opferman, J. T., Ober, B. T. & Ashton-Rickardt, P. G. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745–1748 (1999).
Lanzavecchia, A. & Sallusto, F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290, 92–97 (2000).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Goldrath, A. W. & Bevan, M. J. Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 (1999).
Brocker, T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J. Exp. Med. 186, 1223–1232 (1997).
Author information
Authors and Affiliations
Related links
Rights and permissions
About this article
Cite this article
Gray, D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol 2, 60–65 (2002). https://doi.org/10.1038/nri706
Issue Date:
DOI: https://doi.org/10.1038/nri706
This article is cited by
-
Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies
Immunologic Research (2008)
-
The effector to memory transition of CD4 T cells
Immunologic Research (2008)
-
B-cell memory: are subsets necessary?
Nature Reviews Immunology (2006)
-
The role of models in understanding CD8+ T-cell memory
Nature Reviews Immunology (2005)
-
Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites
Nature Medicine (2004)