Key Points
-
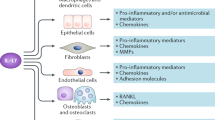
Interleukin-1 (IL-1) is a pro-inflammatory cytokine that plays a role in many acute and chronic human diseases.
-
Elevated levels of IL-1, or the naturally occurring receptor antagonist IL-1Ra, can be measured in human samples of tissue or body fluids from patients with rheumatoid arthritis (RA), osteoarthritis, chronic obstructive pulmonary disease, asthma, inflammatory bowel disease and atherosclerosis.
-
Blockade of IL-1 binding to the IL-1 receptor has been achieved with biological agents that neutralize IL-1. Anakinra, a recombinant form of IL-1Ra, shows clinical efficacy in RA. Other agents show promise in preclinical models and are safe and well tolerated in Phase I volunteer studies.
-
Small-molecule drugs are being developed that interfere with IL-1 signalling production or processing. The most advanced of these is an inhibitor of IL-1-converting enzyme, which has has shown clinical efficacy in RA. Other approaches include targeting the post-translational processing or release of IL-1 and have yielded encouraging results in preclinical studies.
Abstract
The cytokine interleukin-1 (IL-1) is important in many human diseases, and drives a wide range of inflammatory responses in a number of cell types. Biological agents that target IL-1 neutralization have shown efficacy in patients with rheumatoid arthritis. Recently, new classes of compounds that target IL-1 production or processing, or regulatory steps on the IL-1 pathway, are being investigated as new drugs for the management of inflammatory disease. Such compounds will have wide utility in numerous inflammatory conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arend, W. P., Malyak, M., Guthridge, C. J. & Gabay, C. Interleukin-1 receptor antagonist: role in biology. Annu. Rev. Immunol. 16, 27–55 (1998).
Hallegua, D. S. & Weisman, M. H. Potential therapeutic uses of interleukin-1 receptor antagonists in human diseases. Ann. Rheum. Dis. 61, 960–967 (2003).
Cominelli, F. & Pizarro, T. T. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment. Pharmacol. Ther. 10, 49–53 (1996).
Rothwell, N. J. Interleukin-1 and neuronal injury: mechanisms, modification and therapeutic potential. Brain Behav. Immun. 17, 152–157 (2003).
Dayer, J. M. The saga of the discovery of IL-1 and TNF and their specific inhibitors in the pathogenesis and treatment of rheumatoid arthritis. Joint Bone Spine 69, 123–132 (2002).
Chikanza, I. C., Roux-Lombrad, P., Dayer, J. M. & Panayi, G. S. Dysregulation of the in vivo production of interleukin-1 receptor antagonist in patients with rheumatoid arthritis. Pathogenetic implications. Arthritis Rheum. 38, 642–648 (1995).
Casini-Raggi, V. et al. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J. Immunol. 154, 2434–2440 (1995).
Eastgate, J. A. et al. Correlation of plasma interleukin-1 levels with disease activity in rheumatoid arthritis. Lancet 2, 706–708 (1988). A key paper in providing a linkage between the levels of plasma IL-1β and disease score as determined by the Ritchie articular index.
Rooney, M., Symons, J. A. & Duff, G. W. Interleukin-1β in synovial fluid is related to local disease activity in rheumatoid arthritis. Rheumatol. Int. 10, 217–219 (1990).
Dentener, M. A. et al. Systemic anti-inflammatory mediators in COPD: increase in soluble interleukin 1 receptor II during treatment of exacerbations. Thorax 56, 721–726 (2001).
Isaacs, K. L., Sartor, R. B. & Haskill, J. S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology 103, 1587–1595 (1992).
Firestein, G. S. et al. IL-1 receptor antagonist production and gene expression in rheumatoid arthritis and osteoarthritis synovium. Arthritis Rheum. 149, 1054–1062 (1992).
Kahle, P. et al. Determination of cytokines in synovial fluids: correlation with diagnosis and histommorphological characteristics of synovial fluid. Ann. Rheum. Dis. 51, 731–734 (1992).
Koch, A. E., Kunkel, S. L., Chensue, S. W., Haines, G. K. & Streiter, R. M. Expression of interleukin-1 and interleukin-1 receptor antagonist by human rheumatoid synovial tissue macrophages. Clin. Immunol. Immunopathol. 65, 23–29 (1992).
Nouri, A. M., Panayi, G. S. & Goodman, S. M. Cytokines and the chronic inflammation of inflammatory disease. I. The presence of interleukin-1 in synovial fluids. Clin. Exp. Immunol. 55, 295–302 (1984).
Chung, K. F. Cytokines in chronic obstructive pulmonary disease. Eur. Resp. J. Suppl. 34, S50–S59 (2001).
Broide, D. H. et al. Cytokines in symptomatic asthma airways. J. Allergy Clin. Immunol. 89, 958–967 (1992).
Sousa, A. R., Lane, S. J., Nakhosteen, J. A., Lee, T. H. & Poston, R. N. Expression of interleukin-1β (IL-1β) and interleukin-1 receptor antagonist (IL-1Ra) on asthmatic bronchial epithelium. Am. J. Respir. Crit. Care Med. 154, 1061–1066 (1996).
Pomerantz, B. J., Reznikov, L. L., Harken, A. H. & Dinarello, C. A. Inhibition of caspase I reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1β. Proc. Natl Acad. Sci. USA 98, 2871–2876 (2001).
Pearce, W. H., Sweis, I., Yao, J. S., McCarthy, W. J. & Koch, A. E. Interleukin-1β and tumour necrosis factor-α release in normal and diseased human infrarenal aortas. J. Vasc. Surg. 16, 784–789 (1992).
Lombardt, V. R. M., Garcia, M. & Cacabelos, R. R. Characterisation of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy Alzheimer's disease individuals. J. Neuroimmunol. 97, 163–171 (1999).
Griffin, W. S. T. & Mrak, R. E. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J. Leukoc. Biol. 72, 233–238 (2002).
Sheng, J. G., Mrak, R. E. & Griffin, W. S. T. Interleukin-1 expression in brain regions in Alzheimer's disease: correlation with neuritic plaque distribution. Neuropathol. Appl. Neurobiol. 21, 290–301 (1995).
Basu, A. et al. The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J. Neurosci. 22, 6071–6082 (2002).
Calkins, C. M. et al. IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J. Endotoxin Res. 8, 59–67 (2002).
Sims, J. E. IL-1 and IL-18 receptors, and their extended family. Curr. Opin. Immunol. 14, 117–122 (2002).
Smith, D. et al. Four new members expand the interleukin-1 family. J. Biol. Chem. 275, 1169–1175 (2000). Isolation of four new members of the IL-1 superfamily by cDNA cloning from a human placental cDNA library.
Cerretti, D. P. et al. Molecular cloning of the interleukin-1β converting enzyme. Science 256, 97–100 (1992).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 (1992).
Dinarello, C. A. Interleukin-1β, interleukin-18 and the interleukin-1β converting enzyme. Ann. NY Acad. Sci. 856, 1–11 (1998).
Kobayashi, Y. et al. Identification of a calcium-activated neutral protease as a processing enzyme of human interleukin-1α. Proc. Natl Acad. Sci. USA 87, 5548–5552 (1990).
Wakabayashi, G. et al. Staphlococcus epidermidis induces complement activation, tumour necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia — comparison to Escherichia coli. J. Clin. Invest. 87, 1925–1935 (1991).
Greenfeder, S. A. et al. Molecular cloning and characterisation of a second subunit of the interleukin-1 receptor complex. J. Biol. Chem. 270, 13757–13765 (1995).
Liege, S., Laye, S., Li, K., Moze, E. & Neveu, P. J. Interleukin-1 receptor accessory protein (IL-1RacP) is necessary for centrally mediated neuroendocrine and immune responses to IL-1β. J. Neuroimmunol. 110, 134–139 (2000).
Casadio, R. et al. Model of interaction of of the IL-1 receptor accessory protein IL-1RacP with the IL-1β/IL-1RI complex. FEBS Lett. 499, 65–68 (2001).
Smeets, R. L. et al. Effectiveness of the soluble form of the interleukin-1 receptor accessory protein as an inhibitor of interleukin-1 in collagen-induced arthritis. Arthritis Rheum. 48, 2949–2958 (2003).
Burns, K. et al. MyD88, an adapter protein involved in interleukin-1 signalling. J. Biol. Chem. 273, 12203–12209 (1998).
Janssens, S. & Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor associated kinase (IRAK) family members. Mol. Cell 11, 293–302 (2003). A review of the complex molecular signalling cascades that are stimulated by members of the IL-1 superfamily.
Luheshi, G. N. Cytokines and fever. Mechanisms and sites of action. Ann. NY Acad. Sci. 856, 83–89 (1998).
Vincenti, M. P. & Brinckerhoff, C. E. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1β. Arthritis Res. 3, 381–388 (2001). Identification of IL-1β target genes by microarray analysis of human chondrosarcoma cells. This study illustrates the involvement of IL-1β in the temporal regulation of early through to late gene expression.
van den Berg, W. B., Joosten, L. A. & van de Loo, F. A. TNF-α and IL-1β are separate targets in chronic arthritis. Clin. Exp. Rheumatol. 17, S105–S214 (1999).
Mengshol, J. A., Vincenti, M. P., Coon, C. I., Barchowsky, A. & Brinckerhoff, C. E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 43, 801–811 (2000).
McCachren, S. S., Greer, P. K. & Niedel, J. E. Regulation of human synovial fibroblast collagenase messenger RNA by interleukin-1. Arthritis Rheum. 32, 1539–1545 (1989).
Blanco, F. J. & Lotz, M. IL-1 induced nitric oxide inhibits chondrocyte proliferation via PGE2 . Exp. Cell Res. 218, 319–325.
Neumann, D., Kollewe, C., Martin, M. U. & Boraschi, D. The membrane form of the type II receptor accounts for inhibitory function. J. Immunol. 165, 3350–3357 (2000)
Attur, M. G. et al. Functional genomic analysis of type II IL-1β decoy receptor: potential for gene therapy in human arthritis and inflammation. J. Immunol. 168, 2001–2010 (2002).
Bourke, E. et al. Il-1β scavenging by the type II IL-1 decoy receptor in human neutrophils. J. Immunol. 170, 5999–6005 (2003).
Arend, W. P. Interleukin-1 receptor antagonist. Adv. Immunol. 54, 167–227 (1999).
Gouze, J. -N. et al. A comparative study of the inhibitory effects of interleukin-1 receptor antagonist following administration as a recombinant protein or by gene transfer. Arthritis Res. Ther. 5, R301–R309 (2003).
Abramson, S. B. & Amin, A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology 41, 972–980 (2002).
Arend, W. P. & Gabay, C. Physiology of interleukin-1 receptor antagonist. Arthritis Res. 2, 245–248 (2000).
Arend, W. P., Welgus, H. G., Thompson, R. C. & Eisenberg, S. P. Biological properties of recombinant human monocyte-derived interleukin-1 receptor antagonist. J. Clin. Invest. 85, 1694–1697 (1990).
Dinarello, C. The role of interleukin-1 receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 343, 732–734 (2000).
Seckinger, P. et al. Natural and recombinant human IL-1 receptor antagonists block the effects of IL-1 on bone resorption and prostaglandin production. J. Immunol. 145, 4181–4184 (1990).
Ruggiero, P. et al. Inhibitory activity of IL-1 receptor antagonist depends on the balance between binding capacity for IL-1 receptor type I and IL-1 receptor type II. J. Immunology 158, 3881–3887 (1997).
van de Loo, F. A., Joosten, L. A., van Lent, P. L., Arntz, O. J. & van den Berg, W. B. Role of interleukin-1, tumour necrosis factor-α and interleukin-6 in cartilage proteoglycan metabolism and destruction: effect of in situ blocking in murine antigen and zymogen-induced arthritis. Arthritis Rheum. 38, 164–172 (1995).
Joosten, L. A., Helsen, M. M., van de Loo, F. A. & van den Berg, W. B. Anticytokine treatment of established type-II collagen-induced arthritis in DBA/1 mice: a comparitive study using anti-tumour necrosis factor-α, anti-interleukin-1α/β and interleukin-1 receptor antagonist. Arthritis Rheum. 39, 797–809 (1996).
Schwab, J. H., Anderle, S. K., Brown, R. R., Daldorf, F. G. & Thompson, R. C. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis in rats. Infect. Immunol. 59, 4436–4442 (1991).
Wooley, P. H. et al. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 36, 1305–1315 (1993).
Henderson, B., Thompson, R. C., Hardingham, T. & Lewthwaite, J. Inhibition of interleukin-1-induced synovitis and articular cartilage proteoglycan loss in the rabbit knee by recombinant human interleukin-1 receptor antagonist. Cytokine 2, 246–249 (1991).
Matsukawa, A., Ohkawara, S., Maeda, T., Takagi, K. & Yoshinaga, M. Production of IL-1 and IL-1 receptor antagonist and the pathological significance in lipopolysaccharide-induced arthritis in rabbits. Clin. Exp. Immunol. 93, 206–211 (1993).
Roessler, B. J. et al. Inhibition of interleukin-1 induced effects in synoviocytes transduced with the human IL-1 receptor antagonist cDNA using an adenoviral vector. Hum. Gene. Ther. 6, 307–316 (1995).
Bandara, G. et al. Intra-articular expression of biologically active interleukin-1-receptor antagonist protein by ex vivo gene transfer. Proc. Natl Acad. Sci. USA 90, 10764–10768 (1993).
Geiger, T. et al. Neutralization of interleukin-1 activity in vivo with a monoclonal antibody alleviates collagen-induced arthritis in DBA/1 mice and prevents the acute-phase response. Clin. Exp. Rheumatol. 11, 515–522 (1993).
Bakker, A. C. et al. Prevention of murine collagen-induced arthritis in the knee and ipsilateral paw by local expression of human interleukin-1 receptor antagonist protein in the knee. Arthritis Rheum. 40, 893–900 (1997).
Muller-Lander, U. et al. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J. Immunol. 158, 3492–3498 (1997).
Campion, G. V., Lesback, M. E., Lookabaugh, J., Gordon, G. & Catalano, M. A. Dose-range and dose-frequency of study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis. Arthritis Rheum. 39, 1092–1101 (1996). The first safety and tolerability study of anakinra in humans when dosed subcutaneously.
Bresnihan, B. et al. Treatment of rheumatoid arthritis with recombinant interleukin-1 receptor antagonist. Arthritis Rheum. 41, 2196–2204 (1998). A double-blinded, placebo-controlled study of anakinra to patients with active RA on a naive background. Significant clinical findings were reported after this 24-week study was completed.
Jiang, Y. et al. A multi-centre, double-blind, dose ranging, randomised, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation with Genant and Larsen scores. Arthritis Rheum. 43, 1001–1009 (2000).
Bendele, A. et al. Effects of interleukin 1 receptor antagonist alone and in combination with methotrexate in adjuvant arthritic rats. J. Rheumatol. 26, 1225–1229 (1999).
Bendele, A. M. et al. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumour necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 43, 2648–2659 (2000).
Felge, U. et al. Anti-interleukin-1 and anti-tumor necrosis factor-α synergistically inhibit adjuvant arthritis in Lewis rats. Cell. Mol. Life Sci. 57, 1457–1470 (2000).
Cohen, S. et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 614–624 (2002).
Felson, D. T. et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 36, 729–740 (1993).
Dayer, J. M. & Bresnihan, B. Targeting interleukin-1 in the treatment of rheumatoid arthritis. Arthritis & Rheum. 46, 574–548 (2002).
Schiff, M. H. et al. Safety of combination therapy with anakinra and etanercept in patients with rheumatoid arthritis. Arthritis Rheum. 44, S79 (2001).
Bresnihan, B. & Cunnane, G. Infection complications associated with the use of biologic agents. Rheum. Dis. Clin. North Am. 29, 185–202 (2003).
Mohan, A. K., Cote, T. R., Siegel, J. N. & Braun, M. M. Infectious complications of biologic treatments of rheumatoid arthritis. Curr. Opin. Rheumatol. 15, 179–184 (2003). References 77 and 78 are comprehensive reviews tracking the occurrence and frequency of opportunistic infections in patients treated with anti-TNF-α and anti-IL-1 biological agents.
Fleischmann, R. M. et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra) in patients with rheumatoid arthritis. A large, international, multi-centre, placebo-controlled trial. Arthrits Rheum. 48, 927–934 (2003).
Hirsch, E., Irikura, V. M., Paul, S. M. & Hirsch, D. Functions of interleukin-1 receptor antagonist in gene knockout and overproducing mice. Proc. Natl Acad. Sci. USA 93, 11008–11013 (1996).
Fukumoto, T., Matsukawa, A., Ohkawara, S., Takagi, K. & Yoshniaga, M. Administration of neutralizing antibody against rabbit IL-1 receptor antagonist exacerbates lipopolysaccharide-induced arthritis in rabbits. Inflamm. Res. 45, 479–485 (1996).
Ma, Y. et al. Altered susceptibility to collagen induced arthritis in transgenic mice with aberrant expression of IL-1 receptor antagonist. Arthritis Rheum. 41, 1798–1805 (1998).
Bendele, A. et al. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: comparison of efficacy in animal models with human clinical data. Arthritis Rheum. 42, 498–506 (1999). Demonstration of efficacy of IL-1Ra in rat collagen- and adjuvant-induced arthritis models. Data in this paper compare the efficacy and plasma levels of IL-1Ra in both preclinical studies and relate the data to the read out the clinical trial data in humans.
Goupille, P. et al. Safety and efficacy of intra-articular injection of IL-1Ra (IL-1 receptor antagonist) in patients with painful arthritis of the knee: a multi-centre, double blind study. Arthrits Rheum. 48, Suppl. S696. An abstract describing a preliminary study uing anakinra in patients with OA. This may extend the utility of anakinra in OA and further validate IL-1 in joint disease.
Gabay, C. IL-1 trap Regenereon/Novartis. Curr. Opin. Invest. Drugs. 4, 593–597 (2003).
Economides, A. N. et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nature Med. 9, 47–52 (2003). An elegant description of cytokine trap technology, which is widely applicable across cytokines and offers a new approach to ameliorating cytokine-driven disease.
Guler, H. -P. et al. A phase I, single dose escalation study of IL–1 trap in patients with rheumatoid arthritis. Arthritis Rheum. 44, S370 (2001).
Caldwell, J. et al. Results of a phase 1 safety and pharmacokinetic study of interleukin-1 receptor in rheumatoid arthritis. Proc. Eur. League against Rheum. Abstract FR10098 (2002).
Perregaux, D. G. et al. Identification and characterisation of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 299, 187–197 (2001). Discovery of a novel class of compounds that block IL-1β processing and release. Their precise mechanism of action remains unknown.
Perregaux, D. & Gabel, C. A. Interleukin-1β maturation and release in response to ATP and nigericin. J. Biol. Chem. 269, 15195–15203 (1994).
Walev, I., Reske, K., Palmer, M., Valeva, A. & Bhakdi, S. Potassium-inhibited processing of IL-1β in human monocytes. EMBO J. 14, 1607–1614 (1995).
Laliberte, R. E. et al. Glutathione S-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1β post-translational processing. J. Biol. Chem. 278, 16567–16578 (2003).
Ichikawa, K. et al. Novel cytokine production inhibitors produced by a Basidiomycete, Marasmiellus sp. J. Antibiot. 54, 703–709.
Ichikawa, K. et al. LL-Z1271α: an interleukin-1β production inhibitor. Biochem. Biophys. Res. Commun. 286, 697–700 (2001).
Ku, G., Ford, P., Raybuck, S. A., Harding, M. W. & Randle, J. C. R. Selective interleukin-1β converting enzyme (ICE/caspase-1) inhibition with pralnacasan (HMR 3480/VX-740) reduces inflammation and joint destruction in murine type II collagen–induced arthritis (CIA). Arthritis Rheum. 44 (Suppl. 9), S241 (2001).
Pavelka, K. et al. Clinical effects of pralnacasan (PRAL), an orally-active interleukin-1β converting enzyme (ICE) inhibitor in a 285-patient phase II trial in rheumatoid arthritis (RA). Arthritis Rheum. 46 (Suppl. 9), S281 (2002).
Provvedini, D. & Cohen, P. Efficacite de la diacerheine sur les signes fonctionnels et la progression radiologie de l'arthrose. Presse Med. 31, 4S13–4S15 (2002).
Lipsky, P. E. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N. Engl. J. Med. 343, 1594–1602 (2000).
Rudlophi, K., Gerwin, N., Verzijl, N., van der Kraan, P. & van den Berg, W. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarth. Cartil. 11, 738–746 (2003).
Braddock, M. Inflammatory processes in drug discovery — SRI conference. IDrugs 6, 1049–1052 (2003).
Acknowledgements
We thank our colleagues in the Respiratory and Inflammation Research Area and in clinical development within AstraZeneca for their support and stimulating discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
LocusLink
Online Mendelian Inheritance in Man
Glossary
- AUTOCRINE
-
The secretion of a substance, such as a cytokine, that stimulates the secretory cell itself.
- PROXIMAL
-
Nearest the point of origin of a biochemical pathway or molecular process, as opposed to distal, which represents a point towards or at the end of a pathway or process.
- ACUTE-PHASE PROTEINS
-
Antibody-proteins or glycoproteins usually found in the plasma whose concentration increases in response to infection or injury.
- PHARMACOKINETICS
-
The rate by which a drug is absorbed, distributed, metabolized and eliminated by the body.
- PEGYLATED
-
Attachment of non-toxic polyethylene (PEG) polymers to drug and protein molecules to alter their properties to improve safety and efficacy.
Rights and permissions
About this article
Cite this article
Braddock, M., Quinn, A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov 3, 330–340 (2004). https://doi.org/10.1038/nrd1342
Issue Date:
DOI: https://doi.org/10.1038/nrd1342
This article is cited by
-
Potential of oligonucleotide- and protein/peptide-based therapeutics in the management of toxicant/stressor-induced diseases
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Expression and characterization of recombinant IL-1Ra in Aspergillus oryzae as a system
BMC Biotechnology (2023)
-
Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model
Nature Biomedical Engineering (2019)
-
Interleukin-1 receptor type 1 is overexpressed in neurons but not in glial cells within the rat superficial spinal dorsal horn in complete Freund adjuvant-induced inflammatory pain
Journal of Neuroinflammation (2017)
-
Exposures to the environmental toxicants pentachlorophenol (PCP) and dichlorodiphenyltrichloroethane (DDT) modify secretion of interleukin 1-beta (IL-1β) from human immune cells
Archives of Toxicology (2017)