Key Points
-
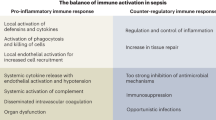
After two decades of clinical trials to find therapeutic treatments for sepsis and its related conditions, activated protein C was recently approved for the treatment of severe sepsis. During these years, much has been learned about how innate immunity functions to control microbial infection, and the pathological consequences that ensue when this response fails.
-
Glucocorticoids are used routinely in a pharmacological capacity because of their potent, broad-spectrum anti-inflammatory action, and they are a mainstay of therapy for many different inflammatory and autoimmune diseases. Many believe that these high doses of glucocorticosteroids suppress innate immune responses and compromise the ability of the host to eliminate the ongoing infection.
-
Immunoneutralization of C5/C5a has been successfully applied in clinical settings of cardiac ischaemia/reperfusion, which portends benefit in severe sepsis, a situation in which hypoperfusion and ischaemia contribute to multi-organ damage.
-
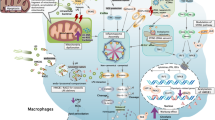
The cytokine macrophage migration inhibitory factor (MIF) is an upstream activator of monocytes/macrophages, and MIF immunoneutralization or genetic deletion in mice results in lower concentrations of tumour-necrosis factor-α, interleukin-1 and prostaglandin E2. Anti-MIF also protects mice from toxic-shock syndrome toxin-1-induced lethality, and MIF knockout mice are resistant to a lethal injection of staphylococcal enterotoxin B. MIF is a promising target.
-
High-mobility group B-1 (HMGB-1) is a nuclear binding protein that circulates at high concentrations relatively late (8–32 hours) after lipopolysaccharide administration to mice. This protein might be an appropriate target for sepsis. A candidate small molecule that improves survival in a preclinical model of sepsis was recently reported to be associated with reduced HMGB-1 production in vivo.
-
Our increased understanding of the complex, interconnected networks involving the inflammatory response, the complement and coagulation pathways, and host metabolic responses might lead to new therapeutics for sepsis.
Abstract
Severe sepsis leading to shock is the principal cause of death in non-cardiac intensive care units. This condition develops because of a dysregulation in host responses, such that the mechanisms initially recruited to fight infection produce life-threatening tissue damage and death. Recent advances in our understanding of innate immunity, and the interaction between the inflammatory and the haemostatic cascades, are affording new opportunities for therapeutic development. This article discusses selected pathophysiological mechanisms that might yield to therapeutic intervention, considers rationales for the resurrection of previously failed drugs, and highlights new targets that have shown promise in preclinical studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Martin, G. S., Mannimo, D. M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Quartin, A. A., Schein, R. M., Kett, D. H. & Peduzzi, P. N. for the Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. Magnitude and duration of the effect of sepsis on survival. JAMA 277, 1058–1063 (1997).
Pert, T. M., Dvorak, L., Hwang, T. & Wenzel, R. P. Long-term survival and function after suspected gram-negative sepsis. JAMA 274, 338–345 (1995).
Bone, R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101, 1644–1655 (1992).
Rangel-Frausto, M. S. et al. The natural history of the systemic inflammatory response syndrome (SIRS): A prospective study. JAMA 273, 117–123 (1995).
Cohen, J. The immunopathogenesis of sepsis. Nature 420, 885–891 (2003).
Young, L. S. in Principles and Practice of Infectious Diseases (eds Mandell, G. L., Bennett, J. E. & Dolin, R.) 806–820 (Churchill Livingstone, New York, 2000).
Angus, D. C. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001).
Janeway, C. A., Travers, P., Walport, M. & Shlomchik, M. Immunobiology 35–91 (Garland, New York, 2001).
Aderem, A. & Ulevitch, R. J. Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000).
Gordon, S. Pattern recognition receptors: doubling up for the innate immune response. Cell 111, 927–930 (2002).
Bouchon, A., Dietrich, J. & Colonna, M. Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 (2000).
Inohara, N., Ogura, Y. & Nunez, G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr. Opin. Microbiol. 5, 76–80 (2002).
Papageorgious, A. C. & Acharya, K. R. Microbial superantigens: from structure to function. Trends Microbiol. 8, 369–375 (2000).
Faust, S. N. Dysfunction of endothelial protein C activation in severe menigococcal sepsis. N. Engl. J. Med. 345, 408–416 (2001).
Esmon, C. T. Regulation of blood coagulation. Biochim. Biophys. Acta 1477, 349–360 (2000).
Rivers, E. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345, 1368–1377 (2001).
Lebbos, J., Bradsher, J. & Kirkpatrick, P. Drotrecogin alpha (activated). Nature Rev. Drug Discov. 2, 13–14 (2003).
Bernard, G. R. et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 (2001).
Matthay, M. A. Severe sepsis — a new treatment with both anticoagulant and anti-inflamamtory properties. N. Engl. J. Med. 344, 759–762 (2001).
Schimmer, B. P. & Parker, K. L. Goodman & Gilman's The Pharmacological Basis of Therapeutics (eds Harmann, J. G. & Limbird, L. E.) 1649–1678 (McGraw Hill, Columbus, 2001).
Lefering, R. & Neugebauer, E. A. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit. Care Med. 23, 1294–303 (1995).
Cronin, L. et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit. Care Med. 23, 1430–1439 (1995).
Annane, D. et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 283, 1038–1045 (2000).
Annane, D. et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. Br. J. Clin. Pharmacol. 46, 589–597 (1998).
Molijn, G. J. et al. Differential adaptation of glucocorticoid sensitivity of peripheral blood mononuclear leukocytes in patients with sepsis or septic shock. J. Clin. Endocrinol. Metab. 80, 1799–1803 (1995).
Annane, D. et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288, 862–871 (2002).
Ziegler, E. J. et al. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N. Engl. J. Med. 307, 1225–1230 (1982).
Baumgartner, J. D. et al. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet 2, 59–63 (1985).
Schedel, I. et al. Treatment of gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit. Care Med. 19, 1104–1113 (1991).
Greenman, R. L. et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA 266, 1097–1102 (1991).
Bone, R. C. et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. The E5 Sepsis Study Group. Crit. Care Med. 23, 994–1006 (1995).
Angus, D. C. et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA 283, 1723–1730 (2000).
The National Committee for the Evaluation of Centoxin. The French National Registry of HA-1A (Centoxin) in septic shock. A cohort study of 600 patients. Arch. Intern. Med. 154, 2484–2491 (1994).
McCloskey, R. V., Straube, R. C., Sanders, C., Smith, S. M. & Smith, C. R. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann. Intern. Med. 121, 1–5 (1994).
Nakamura, T. et al. Hemoperfusion with polymyxin B-immobilized fiber attenuates the increased plasma levels of thrombomodulin and von Willebrand factor from patients with septic shock. Blood Purif. 16, 179–186 (1998).
Ebihara, I., Nakamura, T., Shimada, N., Shoji, H. & Koide, H. Effect of hemoperfusion with polymyxin B-immobilized fiber on plasma endothelin-1 and endothelin-1 mRNA in monocytes from patients with sepsis. Am. J. Kidney Dis. 32, 953–961 (1998).
Nakamura, T. et al. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and β-thromboglobulin. Inflamm. Res. 48, 171–175 (1999).
Bucklin, S. E., Lake, P., Logdberg, L. & Morrison, D. C. Therapeutic efficacy of a polymyxin B–dextran 70 conjugate in experimental model of endotoxemia. Antimicrob. Agents Chemother. 39, 1462–1466 (1995).
Doig, G. S., Martin, C. M. & Sibbald, W. J. Polymyxin–dextran antiendotoxin pretreatment in an ovine model of normotensive sepsis. Crit. Care Med. 25, 1956–1961 (1997).
Warren, H. S. et al. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J. Exp. Med. 177, 89–97 (1993).
Dinarello, C. A. & Wolff, S. M. The role of interleukin-1 in disease. N. Engl. J. Med. 328, 106–113 (1993).
Ruddle, N. H. & Waksman, B. H. Cytotoxic effect of lymphocyte–antigen interactions in delayed hypersensitivity. Science 157, 1060–1062 (1967).
Carswell, E. A. et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA 72, 3666–3670 (1975).
Maini, R. et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 354, 1932–1939 (1999).
Targan, S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. Crohn's Disease cA2 Study Group. N. Engl. J. Med. 337, 1029–1035 (1997).
Baugh, J. & Bucala, R. Mechanisms for modulating TNFα in immune and inflammatory disease. Curr. Opin. Drug Discov. Dev. 4, 635–650 (2001).
Abraham, E. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 25, 556–566 (2002).
Zeni, F., Freeman, B. & Natanson, C. Anti-inflammatory therapies to treat sepsis and septic shock: A reassessment. Crit. Care Med. 25, 1095–1100 (1997).
Fisher, C. J. J. et al. Treatment of septic shock with the tumor necrosis factor receptor Fc fusion protein. N. Engl. J. Med. 334, 1697–1702 (1996).
Nunez-Martinez, O. et al. Reactivation tuberculosis in a patient with anti-TNF-α treatment. Am. J. Gastroenterol. 96, 1665–1666 (2001).
Roth, S. et al. Anti-TNF-α monoclonal antibodies (infliximab) and tuberculosis: apropos of 3 cases. Rev. Med. Intern. 23, 312–316 (2002).
Reinhart, K. & Karzai, W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit. Care Med. 29, 121–125 (2001).
Vincent, J. -L., Sun, Q. & Dunois, M. -J. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin. Infect. Dis. 34, 1084–1093 (2002).
Bevilacqua, M. P. et al. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc. Natl Acad. Sci. USA 83, 4533–4537 (1986).
Conkling, P. R., Greenberg, C. S. & Weinberg, J. B. Tumor necrosis factor induces tissue factor-like activity in human leukemia cell line U937 and peripheral blood monocytes. Blood 72, 128–133 (1988).
Esmon, C. T., Taylor, F. B. Jr & Snow, T. R. Inflammation and coagulation: linked processes potentially regulated through a common pathway mediated by protein C. Thromb. Haemost. 66, 160–165 (1991).
Stouthard, J. M. et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb. Haemost. 76, 738–742 (1996).
Yan, Z. et al. Structural requirements of platelet chemokines for neutrophil activation. Blood 84, 2329–2339 (1994).
Abraham, E. Tissue factor inhibition and clinical trial results of tissue factor pathway inhibitor in sepsis. Crit. Care Med. 28, 31–33 (2000).
Wolfe, R. R., Herndon, D. N., Jahoor, F., Miyoshi, H. & Wolfe, M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N. Engl. J. Med. 317, 403–408 (1987).
Mizock, B. A. Alterations in carbohydrate metabolism during stress: a review of the literature. Am. J. Med. 98, 75–84 (1995).
Malmberg, K. Prospective randomized study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. BMJ 314, 1512–1515 (1997).
Malmberg, K., Norhammar, A., Wedel, H. & Ryden, L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose in Acute Myocardial Infarction (DIGAMI) study. Circulation 99, 2626–2632 (1999).
Van den Berghe, G. et al. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 345, 1359–1367 (2001).
Emanuelli, B. et al. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J. Biol. Chem. 275, 15985–15991 (2000).
Czermak, B. J. et al. Protective effects of C5a blockade in sepsis. Nature Med. 5, 788–792 (1999).
Riedermann, N. C. et al. Increased C5a receptor expression in sepsis. J. Clin. Invest. 110, 101–108 (2002).
Bernhagen, J. et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365, 756–759 (1993).
Calandra, T. et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377, 68–71 (1995).
Bozza, M. et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341–346 (1999).
Calandra, T. et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nature Med. 6, 164–169 (2000).
Mitchell, R. A. et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl Acad. Sci. USA 99, 345–350 (2002).
Roger, T., David, J., Glauser, M. P. & Calandra, T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414, 920–924 (2001).
Calandra, T., Spiegel, L. A., Metz, C. N. & Bucala, R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc. Natl Acad. Sci. USA 95, 11383–11388 (1998).
Beishuizen, A., Thijs, L. G., Haanen, C. & Vermes, I. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J. Clin. Endocrinol. Metab. 86, 2811–2816 (2001).
Joshi, P. C., Poole, G. V., Sachdev, V. Z. X. & Jones, Q. Trauma patients with positive cultures have higher levels of circulating macrophage migration inhibitory factor. Res. Commun. Mol. Pathol. Pharmacol. 107, 13–20 (2000).
Heumann, D. & Glauser, M. P. Anti-cytokine strategies for the treatment of septic shock: relevance of animal models. Curr. Top. Microbiol. Immunol. 216, 299–311 (1996).
Echtenacher, B., Falk, W., Mannel, D. N. & Krammer, P. H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145, 3762–3766 (1990).
Bagby, G. J., Plessala, K. J., Wilson, L. A., Thompson, J. J. & Nelson, S. Divergent efficacy of antibody to tumor necrosis factor-α in intravascular and peritonitis models of sepsis. J. Infect. Dis. 163, 83–88 (1991).
Eskandari, M. K. et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 148, 2724–2730 (1992).
Remick, D. et al. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock 4, 89–95 (1995).
Baugh, J. A. et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 3, 170–176 (2002).
Suzuki, M. et al. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nature Struct. Biol. 3, 259–266 (1996).
Sun, H. W., Bernhagen, J., Bucala, R. & Lolis, E. Crystal structure at 2.6-Å resolution of human macrophage migration inhibitory factor. Proc. Natl Acad. Sci. USA 93, 5191–5196 (1996).
Swope, M., Sun, H. W., Blake, P. R. & Lolis, E. Direct link between cytokine activity and a catalytic site for macrophage migration inhibitory factor. EMBO J. 17, 3534–3541 (1998).
Lubetsky, J. B., Swope, M., Dealwis, C., Blake, P. & Lolis, E. Pro-1 of macrophage migration inhibitory factor functions as a catalytic base in the phenylpyruvate tautomerase activity. Biochemistry 38, 7346–7354 (1999).
Taylor, A. B. et al. Crystal structure of macrophage migration inhibitory factor complexed with (E)-2-fluoro-p-hydroxycinnamate at 1.8 Å resolution: implications for enzymatic catalysis and inhibition. Biochemistry 38, 7444–7454 (1998).
Senter, P. D. et al. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc. Natl Acad. Sci. USA 99, 144–149 (2002).
Lubetsky, J. B. et al. The tautomerase activity of MIF is a potential target for discovery of novel anti-inflammatory agents. J. Biol. Chem. 277, 24976–24982 (2002).
Dios, A. et al. Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity. J. Med. Chem. 45, 2410–2416 (2002).
Leng, L. et al. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197, 1467–1476 (2003).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Bustin, M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 19, 5237–5246 (1999).
Yang, H., Wang, H., Czura, C. J. & Tracey, K. J. HMGB1 as a cytokine and therapeutic target. Endotoxin. Res. 8, 469–472 (2002).
Ulloa, L. et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl Acad. Sci. USA 19, 12351–12356 (2002).
Cross, A. S. & Opal, S. M. A new paradigm for the treatment of sepsis: is it time to consider combination therapy? Ann. Intern. Med. 138, 502–505 (2003).
Marshall, J. C. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nature Rev. Drug Discov. 2, 391–405 (2003).
Ebong, S. et al. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect. Immun. 67, 6603–6610 (1999).
Eichacker, P. Q. et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am. J. Respir. Crit. Care Med. 166, 1197–1205 (2002).
Cooke, G. S. & Hill, A. V. Genetics of susceptibility to human infectious disease. Nature Rev. Genet. 2, 967–977 (2001).
Stuber, F., Petersen, M., Bokelmann, F. & Schade, U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-α concentrations and outcome of patients with severe sepsis. Crit. Care Med. 24, 381–384 (1996).
Lorenz, E., Mira, J. P., Frees, K. L. & Schwartz, D. A. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch. Intern. Med. 162, 1028–1032 (2002).
Picard, C., Puel, A., Bonnet, M., Ku, C. L. & Bustamante, J. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299, 2076–2079 (2003).
Bochud, P. Y., Hawn, T. R. & Aderem, A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 170, 3451–3454 (2003).
Acknowledgements
We thank the participants of the 2003 Keystone Symposium: The Molecular and Cellular Basis of Septic Shock (6–9 March 2003, Lake Tahoe, California) for providing a valuable update to many of the concepts discussed in this article. The authors acknowledge research support from the Arthritis Foundation and the National Institutes of Health.
Author information
Authors and Affiliations
Glossary
- INDWELLING DEVICE
-
A device that is invasive, and is associated with risk of infection.
- LEUKOCYTOSIS
-
An increase in leukocyte number in blood, generally caused by infection.
- TACHYPNEA
-
An abnormally rapid respiratory rate.
- ZYMOSAN
-
An insoluble carbohydrate from the cell wall of yeast.
- LECTIN
-
A protein which specifically binds carbohydrates.
- TOXIC-SHOCK SYNDROME
-
A cluster of symptoms that involve many systems of the body, caused by Staphylococcus aureus or Streptococcus pyognenes.
- SUPERANTIGENS
-
Molecules produced by microbes that act independently to stimulate T-cell activities, including cytokine release.
- ENDOGENOUS PYROGEN
-
A cytokine that can induce a rise in body temperature.
- INFLAMMATORY ARTHRITIS
-
A form of arthritis, in which the membrane that lines the joint (the synovial membrane) becomes inflamed, producing pain and swelling.
- INFLAMMATORY BOWEL DISEASE
-
(IBD). This term refers to ulcerative colitis and Crohn's disease, both incurable chronic diseases of the intestinal tract. The two diseases are often grouped together under the rubric of IBD because of their similar symptoms.
- APACHE II
-
Systems to estimate the risk of hospital death based on severity of disease scoring was first introduced with Acute Physiology And Chronic Health Evaluation (APACHE) in 1981 followed by the Simplified Acute Physiology Score (SAPS) in 1988. Further research resulted in 'improved' editions, APACHE II in 1985, and SAPS II in 1993. APACHE III is now coming into general use.
- CACHEXIA
-
A catabolic, wasting state, that is often a consequence of chronic infection or malignancy
Rights and permissions
About this article
Cite this article
Lolis, E., Bucala, R. Therapeutic approaches to innate immunity: severe sepsis and septic shock. Nat Rev Drug Discov 2, 635–645 (2003). https://doi.org/10.1038/nrd1153
Issue Date:
DOI: https://doi.org/10.1038/nrd1153
This article is cited by
-
CircANKRD36 Knockdown Suppressed Cell Viability and Migration of LPS-Stimulated RAW264.7 Cells by Sponging MiR-330
Inflammation (2021)
-
In focus in HCB
Histochemistry and Cell Biology (2019)
-
Bone marrow-derived mesenchymal stem cells ameliorate liver injury in a rat model of sepsis by activating Nrf2 signaling
Histochemistry and Cell Biology (2019)
-
Genome-wide association study reveals a QTL and strong candidate genes for umbilical hernia in pigs on SSC14
BMC Genomics (2018)
-
Dimethyl Fumarate Modulates Oxidative Stress and Inflammation in Organs After Sepsis in Rats
Inflammation (2018)