Abstract
A significant minority of individuals engages in escalated levels of aggression after consuming moderate doses of alcohol (Alc). Neural modulation of escalated aggression involves altered levels of serotonin (5-HT) and the activity of 5-HT1B receptors. The aim of these studies was to determine whether 5-HT1B receptors in the dorsal raphé (DRN), orbitofrontal (OFC), and medial prefrontal (mPFC) cortex attenuate heightened aggression and regulate extracellular levels of 5-HT. Male mice were trained to self-administer Alc by performing an operant response that was reinforced with a delivery of 6% Alc. To identify Alc-heightened aggressors, each mouse was repeatedly tested for aggression after consuming either 1.0 g/kg Alc or H2O. Next, a cannula was implanted into either the DRN, OFC, or mPFC, and subsets of mice were tested for aggression after drinking either Alc or H2O prior to a microinjection of the 5-HT1B agonist, CP-94,253. Additional mice were implanted with a microdialysis probe into the mPFC, through which CP-94,253 was perfused and samples were collected for 5-HT measurement. Approximately 60% of the mice were more aggressive after drinking Alc, confirming the aggression-heightening effects of 1.0 g/kg Alc. Infusion of 1 μg CP-94,253 into the DRN reduced both aggressive and motor behaviors. However, infusion of 1 μg CP-94,253 into the mPFC, but not the OFC, after Alc drinking, increased aggressive behavior. In the mPFC, reverse microdialysis of CP-94,253 increased extracellular levels of 5-HT; levels decreased immediately after the perfusion. This 5-HT increase was attenuated in self-administering mice. These results suggest that 5-HT1B receptors in the mPFC may serve to selectively disinhibit aggressive behavior in mice with a history of Alc self-administration.
Similar content being viewed by others
INTRODUCTION
Alcohol (Alc), more than any other drug, has been associated with acts of violence and aggression. Importantly, in certain situations, Alc has the ability to facilitate aggressive actions. It has been repeatedly demonstrated, in multiple species, that some individuals are sensitive to the aggression-heightening effects of moderate doses of Alc, while most are not (Chance et al, 1973; Peeke et al, 1973; Miczek and O'Donnell, 1980; Lister and Hilakivi, 1988; Miczek et al, 1993, 2004b). Although the neurobiological basis for the differential response to Alc is not yet known, there is compelling evidence for a significant role of the serotonin (5-HT) system in the modulation of impulsive aggressive behavior (Olivier et al, 1989; Olivier and Mos, 1992; Miczek et al, 2004a).
There is a long-standing 5-HT deficiency hypothesis that proposes that basal measures of both peripheral and central 5-HT activity are inversely correlated with indices of aggression and impulsivity in rodents and primates leading to the suggestion that blunted serotonergic activity might be an important factor contributing to the expression of Alc-heightened aggression (Garattini et al, 1967; Giacalone et al, 1968; Coccaro, 1992; Virkkunen and Linnoila, 1993; Mehlman et al, 1994; Higley et al, 1996; van der Vegt et al, 2001). In vivo microdialysis has been used as a more sensitive tool to temporally assess this relationship. In rats, extracellular levels of 5-HT in the nucleus accumbens decrease in anticipation of an aggressive encounter while cortical 5-HT levels decrease during and after an aggressive confrontation (van Erp and Miczek, 2000; Ferrari et al, 2003). These studies reveal that 5-HT levels in specific brain regions serve to regulate distinct phases of aggressive behavior and perhaps the prefrontal cortex may be most involved in the execution of and recovery from an aggressive encounter (van Erp and Miczek, 2000; Halász et al, 2006; Miczek and Fish, 2006).
The influence of the 5-HT1 family of receptors on aggressive behavior has been extensively studied and has revealed that 5-HT1 receptor agonists can dose dependently reduce aggression alone and in the presence of Alc (Olivier and Mos, 1986; Olivier et al, 1995; Miczek et al, 1998; Fish et al, 1999; de Boer and Koolhaas, 2005; Olivier and Van Oorschot, 2005; de Almeida et al, 2006). The 5-HT1B receptor is of particular interest because of the behaviorally specific anti-aggressive effects of receptor-selective 5-HT1B agonists although the effectiveness of one of the most potent 5-HT1B agonists, CP-94,253, depends on the type of aggressive behavior being studied and the route of administration. Specifically, systemic administration of CP-94,253 reduces several forms of escalated aggressive behavior including Alc-, instigation-, and schedule-heightened aggression (Fish et al, 1999, 2007; de Almeida et al, 2006; Bannai et al, 2007). However, the effects of local administration of CP-94,253 into the orbitofrontal cortex (OFC) are more complex and reveal that maternal-instigated aggression is insensitive to microinjection of CP-94,253 while species-typical aggression is potently reduced (de Almeida et al, 2006; Veiga et al, 2007). Together, these studies suggest that escalated and species-typical aggression may share similar but not identical mechanisms and that the prefrontal cortex and 5-HT1B receptors may importantly contribute to these differences.
There is a similar dissociation between anti-aggressive effects of CP-94,253 and its effects on 5-HT levels in the brain. Given systemically, CP-94,253 significantly reduces extracellular levels of 5-HT in the striatum, hippocampus, and prefrontal cortex (Knobelman et al, 2000; Johnson et al, 2001; De Groote et al, 2003; Miczek et al, 2004a). According to the 5-HT deficiency hypothesis, this decrease in 5-HT should be associated with increased aggression, not behaviorally specific anti-aggressive effects. These behavioral effects suggest that the modulation of aggression by 5-HT1B receptors is not solely due to autoreceptor stimulation. An anatomically more discrete examination of alternate 5-HT1B receptor populations is required to understand how this receptor regulates aggression.
The 5-HT1B receptor is highly expressed in brain regions related to aggressive and impulsive behavior, particularly, the prefrontal cortex (PFC) (Hoyer et al, 1985; Bruinvels et al, 1993, 1994; Sari et al, 1999; Sari, 2004). 5-HT neurons project from the dorsal raphé (DRN) to the PFC and there has been increasing evidence that dysfunctions in these neurons may underlie impulsive behavior and aggression (Grafman et al, 1996; Bechara et al, 2000; Brower and Price, 2001; Chudasama et al, 2003; Best et al, 2002; Spinella, 2004). For these reasons, presynaptic 5-HT1B receptors located in this region might be important modulators of Alc-heightened aggression.
The objectives of these experiments were twofold. First, we asked whether 5-HT1B receptors located in the medial prefrontal cortex (mPFC), OFC, or DRN are differentially modulating Alc-heightened aggression using site-specific microinjection of CP-94,253 prior to an aggressive confrontation. Second, we investigated the neurochemical effect of CP-94,253 in the mPFC by quantifying extracellular levels of 5-HT in both Alc-naïve and Alc self-administering mice.
MATERIALS AND METHODS
Subjects
‘Residents’ were 5-week-old male CFW mice (Carworth Farm Webster; Charles River Laboratories, Wilmington, MA), weighing 21–23 g upon arrival, pair-housed with a female in clear, polycarbonate cages (28 × 17 cm) lined with pine shavings. Purina rodent chow was freely available through the cage lid and water was given for 3 h daily. ‘Intruders’ were male CFW mice (n=144) housed 8–12 per large cage (48 × 26 cm) lined with corn cob bedding, with unlimited access to food and water. The vivarium maintained a 12-h light/dark photocycle (lights off at 0700 h), a 21±1°C temperature, and 23% humidity. All mice were cared for according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The Tufts University IACUC approved all experiments.
Alcohol Self-Administration
A custom designed aluminum panel (16.5 × 15.9 cm) was placed into the resident's home cage and secured by two thumbnail screws (Miczek and de Almeida, 2001). Each side contained a cue light positioned above a nose-poke operandum containing a drinking trough (3 × 5 cm; Med Associates, Georgia, VT). Each trough was connected to a syringe pump (Med Associates). The panel and pump were interfaced with a computer controlling the experimental events and recording responses (MED-PC for Windows, v. 4.1; Med Associates). A house light at the top of the panel was illuminated throughout the session. A nose-poke was recorded when the mouse broke a photobeam spanning the operandum. Every fifth response (fixed ratio 5) was reinforced by the delivery of an Alc solution and the secondary cues of an audible click and absence of the house light. A modified sucrose-fading procedure facilitated Alc self-administration (described in Miczek and de Almeida, 2001). Drinking sessions occurred 5 days per week between 0800 and 1400 h.
Resident–Intruder Confrontations and Alcohol-Heightened Aggression
After 3 weeks of pair-housing, the 8-week-old residents were screened for aggression until stable frequencies of attack bites were maintained (approximately 10 confrontations; Miczek and O'Donnell, 1978). Confrontations lasted for 5 min after the first attack bite or 5 min with no attack. To characterize Alc-heightened and non-heightened aggressors, aggression was assessed 15 min after consuming either 1.0 g/kg Alc or water (three tests each) in an alternating sequence. On test days, aggression was tested only if the mouse drank 1.0 g/kg Alc in less than 10 min. Testing occurred three times per week separated by at least 48 h. All confrontations were video-recorded and analyzed by a trained observer (intra-observer reliability: r2=0.97) using The Observer software (Noldus, v.5.0; Wageningen, The Netherlands). The frequencies and durations of aggressive (attack bites, sideways threat, tail rattles, pursuits) and nonaggressive (grooming, rearing, walking) behaviors were quantified following the descriptions of Grant and Mackintosh (1963) and Miczek and O'Donnell (1978).
Experiment 1: Intra-Raphé Microinjections and Aggression
After characterizing Alc-heightened aggression, residents (n=16) were anesthetized with Avertin© (2,2,2 tribromoethanol; Sigma; 400 mg/kg, i.p. ), placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA), and implanted with a 26-gauge guide cannula (Plastics One, Roanoke, VA) aimed toward the DRN (AP, −4.4 mm; ML, ±0; DV, −1.7 mm from dura; interaural, −0.6 mm, after Paxinos and Franklin (2001)). A 33-gauge obdurator (Plastics One), extending 0.5 mm beyond the cannula tip, was inserted after surgery and moved daily to prevent blockage and scarring. An aversive-tasting polish (Bite It©) coated the headmount and obdurator to prevent gnawing damage by the female cagemate. After 1- to 2-week recovery, residents resumed Alc self-administration and aggression testing.
On test days, mice consumed water or 1.0 g/kg Alc immediately before microinjection of artificial cerebrospinal fluid (aCSF), 1.0 μg (+)8-OH-DPAT (5-HT1A agonist), or 1.0 μg CP-94,253 (5-HT1B agonist). The obdurator was removed and a 33-gauge injector was (Plastics One) inserted to extend 2 mm beneath the guide. Flared polyethylene tubing connected the injector to a glass syringe and pump (CMA Microdialysis, North Chelmsford, MA) that infused 0.5 μl over 4 min (0.125 μl/min). The injector remained in place for 1 min after the infusion to allow diffusion and minimize vertical capillary action along the injection tract. Mice were unrestrained during the infusion. Aggression was tested 10 min after the microinjection. A total of six tests were conducted in a randomized sequence and separated by at least 4 days.
After the final aggression test, mice were deeply anesthesized (Avertin®) and intracardially perfused with 0.9% saline and 4% paraformaldehyde. To verify implant position, the brains were sliced on a sliding microtome in 60 μm coronal sections, and stained with cresyl violet (Figure 1a and b). Mice with inaccurate placements or clogged cannulae (n=5) were anatomical controls and excluded from the final analysis.
Distribution of microinjection sites. (a, c, e) A schematic representation of mouse dorsal raphé (DRN), medial prefrontal (mPFC), and orbitofrontal (OFC) cortex coronal sections adapted from Paxinos and Franklin. Circles indicate the approximate site of an accurately placed injection aimed at AP, −4.4 mm; ML, ±0 mm; DV, −1.7 mm; AP, +1.7 mm; ML, ±0.4 mm; DV, −1.2 mm; and AP, +2.5 mm; ML, ±0.7 mm; DV, −1.0 mm, respectively. Triangles represent missed placements. Filled circles represent anatomical sites into which a 1 μg infusion of CP-94,253 effectively reduced (>40% decrease) species-typical aggression (a) and effectively increased (>40% increase) Alc-heightened aggression (c). (b, d, f) Representative mouse brain coronal sections ( × 20) that were stained with cresyl violet to visualize the injection sites in the DRN, mPFC, and OFC, respectively. The black horizontal bar represents 1 mm.
Experiment 2: Medial Prefrontal Cortex Microinjections and Aggression
A second group of residents (n=18) was implanted with a cannula (Plastics One) aimed at either the right or the left medial prefrontal cortex (mPFC: AP, +1.7 mm; ML, ±0.4 mm; DV, −1.2 mm from dura; Figure 1c and d). They were tested for aggression 10 min after consuming water or 1.0 g/kg Alc and a microinjection of either 0.5 μg aCSF or 1 μg CP-94,253. Histological verification of cannula placement revealed that six missed placements were within the lateral septum; these were analyzed independently (data not shown). Two mice had clogged cannula and were excluded from the study.
Experiment 3: Orbitofrontal Cortex Microinjections and Aggression
A third group of residents (n=18) was implanted with a cannula (Plastics One) aimed at either the right or the left orbitofrontal cortex (OFC: AP, +2.5 mm; ML, ±0.7 mm; DV, −1.0 mm from dura). Mice were tested for aggression 10 min after consuming water or 1.0 g/kg Alc, and a microinjection of either 0.5 μg aCSF or 1 μg CP-94,253. The final analysis included 13 residents with accurate OFC injections; three mice died after surgery and two had cannulae placements outside the OFC (Figure 1e and f).
Experiment 4: Measurement of Extracellular 5-HT in the Medial Prefrontal Cortex
A group of experimentally naïve mice (n=5) and a group of mice (n=5) that drank 1.0 g/kg Alc daily (see Alc self-administration for details) were implanted with a CMA/7 guide cannula (CMA Microdialysis) aimed 2 mm above the mPFC (AP, +1.7 mm; ML, +0.3 mm from bregma; DV, −1.5 mm from skull surface) and given at least 1 week to recover.
On the evening before sample collection, mice were anesthesized using isoflurane inhalation anesthesia (AErrane®; Baxter, IL) and a CMA/7 microdialysis probe (membrane length, 2 mm) was inserted into the guide cannula. Fluorinated ethylene polymer tubing (CMA Microdialysis) connected the probe to the single-channel swivel (Instech, Plymouth Meeting, PA), liquid switch (CMA/1100), and syringe pump (CMA/102). The swivel arm allowed free 360° movement of the tethered mouse in its home cage. The probe was perfused with aCSF at a rate of 0.4 μl/min overnight and increased to 0.8 μl/min 1 h before sample collection. Samples were collected every 20 min, and stored at −80°C for future analysis. Three 1-h collection periods (total of nine samples) occurred for baseline, the addition of CP-94,253 (1 μM) to the perfusate and the removal of CP-94,253 from the perfusate. The mice were anesthetized with Avertin® and intracardially perfused to verify probe placement (Supplementary Figure 1).
Serotonin was analyzed by high-performance liquid chromatography (HPLC). Ten-microliter samples were injected onto an Inersil ODS-3 microbore column (3 μm, 1 × 150 mm; LC PACKINGS, Amsterdam, Netherlands) connected to a manual injector (model 7725i; Rheodyne, Cotati, CA) with a 20 μl sample loop and an LC-10ADVP pump (Shimadzu, Kyoto, Japan). An electrochemical detector (VT-03 micro flow cell; ANTEC Leyden, Zoeterwoude, Netherlands) was set at a potential of 600 mV against an Ag/AgCl reference electrode. The signals were detected and analyzed using Control and ChromoGraph Report software (Bioanalytical Systems, West Lafayette, IN), respectively. The mobile phase (25 mM NaH2PO4, 50 mM sodium citrate, 27 μM Na2EDTA, and 2.2 mM 1-octanosulfonic acid, 8% MeOH, pH 4.2) was pumped at a flow rate of 80 μl/min. A standard curve for 5-HT (Fluka; Sigma-Aldrich, St Louis, MO) was generated every day and mouse samples were stored at −80°C and analyzed within 24 h. Peak heights for 5-HT quantified the concentration within each sample.
Drugs
Ethyl alcohol (95%) (Pharmco Products Inc., Brookfield, CT) was diluted with tap water to 10% (w/v). The 5-HT1A agonist, (+)8-OH-DPAT (8-hydroxy-[dipropyl-n-amine] tetralin; Research Biochemicals International, Natick, MA) and the 5-HT1B agonist, CP-94,253 (3-[1,2,5,6-tetrahydro-4-pyridyl]-5-propoxypirolo[3,2-b]pyridine, generously donated by Pfizer, Groton, CT) were freshly dissolved in aCSF (in mM, 147 NaCl, 1.3 anhydrous CaCl2, 0.9 anhydrous MgCl2, 4.0 KCl, pH=6.7–7).
Statistical Analysis
The frequencies of the aggressive behaviors and the durations of the non-aggressive behaviors were analyzed using two-way repeated-measures ANOVA. The Holm-Sidak post-hoc test was run when appropriate, using the aCSF and water tests as the control conditions.
After verifying no systematic trends, baseline levels of 5-HT were quantified by averaging the three baseline samples for each individual mouse. 5-HT levels for the subsequent six samples were expressed as a percent of baseline for each subject. For analysis, a two-way between-subject ANOVA was performed followed by the Holm-Sidak post-hoc test, using the average baseline level of 5-HT and naive mice as the common controls. α was set at 0.05 for all analyses.
RESULTS
The most striking finding from these experiments is the very high level of aggression observed after drug microinjection. In all the experiments, aggressive behavior was significantly increased after microinjection of aCSF and self-administration of 1.0 g/kg Alc, even in those subjects who were previously characterized as ‘alcohol non-heightened aggressors’ prior to surgery. Because of their similar behavioral response to Alc during the microinjection phase of the experiments, Alc-heightened and non-heightened aggressors were analyzed as a single experimental group.
Experiment 1: Intra-Raphé Microinjections and Aggression
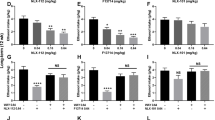
Intra-raphé administration of the prototypic 5-HT1A agonist, 8-OH-DPAT, decreases both species-typical and maternal aggression in rats (Mos et al, 1993; de Almeida and Lucion, 1997). This finding was extended to Alc self-administering mice and to a 5-HT1B receptor agonist. Accordingly, there was a significant main effect of fluid consumption on the frequency of attack bites (F(1, 20)=21.05, p<0.001) and sideways threats (F(1, 20)=16.95, p=0.002). Post-hoc tests revealed that this effect was specifically due to heightened levels of aggressive behavior after self-administration of 1.0 g/kg Alc, regardless of the drug that was microinjected (Figure 2a; Table 1). In addition, a significant main effect of drug was found on the frequency of attack bites (F(2, 20)=9.45, p=0.001) and sideways threats (F(2, 20)=9.4, p=0.001). Both 1 μg (+)8-OH-DPAT and 1 μg CP-94,253 significantly decreased the frequency of attack bites while the frequency of sideways threat was only affected by infusion of 1 μg CP-94,253. Finally, microinjection of 1 μg (+)8-OH-DPAT effectively reduced the frequency of tail rattles (F(2, 20)=9.46, p=0.001). Microinjection sites that effectively reduced the frequency of attack bites after water drinking and 1-μg CP-94,253 infusion compared to water drinking and aCSF infusion by at least 40% are indicated in Figure 1a with closed circles.
Microinjection of 5-HT1 agonists into the dorsal raphé after water or Alc consumption. (a) The effect of the aCSF vehicle (open bars), 8-OH-DPAT (filled gray bars), or CP-94,253 (filled black bars) on the mean (±SEM, vertical lines) frequency of attack bites after the consumption of water (+H2O, left side) or 1.0 g/kg Alc (+1.0 Alc, right side). (b) The effects of these drugs on the duration of walking (in seconds). *A significant decrease from the aCSF vehicle; #a significant main effect of Alc self-administration. p<0.05 for all comparisons.
A significant main effect on the duration of walking was observed (F(1, 20)=5.69, p=0.038). Post-hoc analysis revealed that, overall, mice were more active after self-administration of 1.0 g/kg Alc relative to water (Figure 2b; Table 1). The main effect of drug was obtained for the duration of grooming (F(2, 20)=4.52, p=0.024). Specifically, microinjection of 1 μg CP-94,253 reduced the duration of grooming by 32%.
Experiment 2: Medial Prefrontal Cortex Microinjections and Aggression
As in the first experiment, levels of aggression were significantly elevated after self-administration of 1.0 g/kg Alc regardless of drug treatment (F(1, 18)=7.55, p=0.023; Figure 3a). In addition, there was a significant interaction between self-administered fluid and drug dose, and post-hoc tests revealed that after Alc self-administration, microinjection of 1.0 μg CP-94,253 significantly increased the frequency of attack bites during the 5-min confrontation as compared to the level after aCSF microinjection (p=0.004; Figure 3a). Microinjection sites that effectively increased the frequency of attack bites after ethanol drinking and 1 μg CP-94,253 infusion compared to ethanol drinking and aCSF infusion by at least 40% are indicated in Figure 1c with closed circles.
Microinjection of the 5-HT1B receptor agonist CP-94,253 into the prefrontal cortex after water or Alc consumption. (a) The effects of medial prefrontal microinjection and (b) the effects of orbitofrontal microinjection of the aCSF vehicle (open bars) and the 5-HT1B agonist CP-94,253 at the 0.5 (filled gray bars) and 1.0 μg doses (filled black bars) on the mean (±SEM, vertical lines) frequency of attack bites after the consumption of water (+H2O, left side) or 1.0 g/kg Alc (+1.0 Alc, right side). (c and d) The effects of these treatments on the duration of walking (in seconds) in these two regions, respectively. *A significant decrease from the aCSF vehicle; #a significant main effect of Alc self-administration. p<0.05 for all comparisons.
A significant main effect of drug dose (F(2, 18)=5.03, p=0.018; Figure 3c) as well as a significant interaction (F(2, 18)=3.29, p=0.018) was found for the duration of walking. These results indicate that, while microinjection of 1 μg CP-94,253 alone is not sufficient to modulate motor activity, when paired with self-administration of 1.0 g/kg Alc, the effects on motor activity are additive and result in locomotor hyperactivity. Similarly, a significant main effect of self-administered fluid (F(1, 18)=5.5, p=0.044) and an interaction (F(2, 18)=3.92, p=0.039) were found for grooming duration. Post-hoc analysis revealed that this interaction was due to a significant decrease in the duration of grooming after consuming water and microinjection with 0.5 μg CP-94,253 (Table 2).
Analysis of the mice with placements in the lateral septum revealed a higher frequency of attack bites (F(1, 4)=28.05, p=0.006) and tail rattles (F(1, 4)=16.61, p=0.015) after Alc drinking compared to water self-administration (Figure 4) without a significant interaction between self-administered fluid and drug dose. In addition, walking duration was significantly increased after Alc self-administration irrespective of drug dose (F(1, 4)=13.59, p=0.021; Figure 4).
Microinjection of the 5-HT1B receptor agonist CP-94,253 into the lateral septum after water or Alc consumption. (a) The effect of the aCSF vehicle (open bars) and 1.0 μg CP-94,253 (filled black bars) on the mean (±SEM, vertical lines) frequency of attack bites after the consumption of water (+H2O, left side) or 1.0 g/kg Alc (+1.0 Alc, right side). (b) The effects of these treatments on the duration of walking (in seconds). #A significant main effect of Alc self-administration. p<0.05 for all comparisons.
Experiment 3: Orbitofrontal Cortex Microinjections and Aggression
As in the previous two experiments, there was a significant main effect of self-administered fluid on the frequency of attack bites (F(1, 24)=14.91, p=0.002) and sideways threats (F(1, 24)=15.31, p=0.002; Figure 3b, Table 3). Post-hoc analysis revealed that these measures of aggressive behavior were significantly increased after Alc relative to water self-administration, independent of drug microinjection.
The only significant main effect of drug was found for rearing duration (F(2, 24)=4.79, p=0.018; Table 3). Post-hoc analysis revealed that 1.0 μg CP-94,253 significantly increased the duration of rearing relative to aCSF microinjection.
Experiment 4: Measurement of Extracellular 5-HT in the Medial Prefrontal Cortex
There were significant main effects of drinking history (F(1, 56)=22.80, p<0.001), time (F(6, 56)=11.24, p<0.001), and a significant interaction between both factors (F(6, 56)=2.80, p=0.019) on the levels of extracellular 5-HT in the mPFC (Figure 5). 5-HT levels in both groups of mice were significantly lower 160–200 min after the start of the session, during recovery from the reverse perfusion of CP-94,253. In addition, mice with a history of Alc self-administration showed a blunted neurochemical response to CP-94,253 during the reverse perfusion.
Cortical levels of 5-HT measured during reverse perfusion of 5-HT1B receptor agonist CP-94,253. Circles reflect the mean (±SEM, vertical lines) change in extracellular level of 5-HT in a 20-min sample in Alc-naïve mice and triangles reflect the mean (±SEM, vertical lines) change in extracellular level of 5-HT in a 20-min sample in chronic AlcAlc drinkers. All data are expressed as a percent change from baseline. Filled symbols reflect samples that were collected during the reverse perfusion of 1 μM CP-94,253. The dashed line denotes 100%. *A significant difference between treatment groups at that time point; #a significant change from baseline. p<0.05 for all comparisons.
DISCUSSION
This series of experiments demonstrates that the expression of Alc-heightened aggression is functionally regulated specifically by 5-HT1B receptor activity in the mPFC but not in the OFC or the DRN. Furthermore, the potentiation of Alc-heightened aggression that is observed after local infusion of CP-94,253 into the mPFC may be due to blunted cortical levels of 5-HT in Alc self-administering mice. This pattern of results contrasts with the critical role of the orbito ventral region of the prefrontal cortex in other forms of escalated aggression and suggests that the mechanism underlying Alc-heightened aggression may differ from that of other types of escalated aggressive behavior (de Almeida et al, 2006; Bannai et al, 2007).
These are the first studies that provide evidence toward the respective roles of pre- and post-synaptic 5-HT1B receptors in modulating Alc-heightened and species-typical aggression. The attenuation of species-typical aggression by intra-raphé microinjection of (+)8-OH-DPAT (experiment 1) corroborates the earlier findings of Mos et al (1993) and de Almeida and Lucion (1997) and highlights the importance of somatodendritic 5-HT1 receptors in the modulation of species-typical aggression. This experiment extends this research by showing that local activation of DRN 5-HT1B receptors produces a similar behavioral effect to 5-HT1A receptor activation (ie, attenuation of aggression), coupled with modest reductions in motor behavior. The slight slowing of motor activity seen after CP-94,253 injection was somewhat surprising, given the absence of this effect on species-typical aggression when the drug is systemically administered, but not entirely unexpected because the effect of CP-94,253 on motor activity is dependent on the experimental conditions in which it is administered (Fish et al, 1999, 2007). Nonetheless, the results from experiment 1 suggest that activation of DRN 5-HT1B receptors leads to a nonspecific reduction in non-heightened aggressive behavior. This conclusion is concordant with a recent study showing that intra-raphé microinjection of the 5-HT1B agonist, CP-93,129, nonspecifically decreases schedule-heightened levels of aggression (Bannai et al, 2007). Since 5-HT1 agonists infused directly into the DRN produce a generalized decrease in 5-HT release in all terminal regions of the brain, the function of decreased extracellular 5-HT could be to inhibit motor activity via decreased 5-HT availability in terminal regions that are dopamine-rich and important for regulating motor activity, such as the dorsal striatum (Adell et al, 1993; Adell et al, 2001). Thus, the behavioral specificity of CP-94,253 that occurs after systemic administration, in contrast to the 5-HT1A agonist (+)8-OH-DPAT, could be due to joint stimulation of somatodendritic and post-synaptic 5-HT1B receptors by CP-94,253 vs stimulation of primarily somatodendritic receptors by low doses of (+)8-OH-DPAT.
It is significant that the expression of Alc-heightened aggression was insensitive to intra-raphé infusion of either of these agonists in light of the recent report by Bannai et al (2007) showing a significant reduction in schedule-heightened aggression by intra-raphé infusion of a different 5-HT1B agonist, CP-93,129. Both CP-94,253 and CP-93,129 have a high affinity for the 5-HT1B receptor but differ in their relative affinities for the 5-HT1B receptor vs the 5-HT1A receptor (Ki for 5-HT1A : 5-HT1B=44.5 and 27.27 nM, respectively; Koe et al, 1992a, 1992b; Perez et al, 1998; Pineyro and Blier, 1999). The greater relative affinity of CP-93,129 for the 5-HT1A receptor may account for why this ligand was able to effectively reduce heightened aggressive behavior in contrast to CP-94,253 when infused into a region abundant with 5-HT1A receptors. Nonetheless, the resistance of Alc-heightened aggression to modulation by both (+)8-OH-DPAT and CP-94,253 does indicate that this form of escalated aggression is not under direct modulation by the DRN and that the acute, pro-aggressive effect of 1.0 g/kg Alc is sufficient to counteract the antiaggressive effects of these ligands (Miczek et al, 1998; Fish et al, 1999).
Both impulsive behavior and aggression have been linked to dysfunctions of the prefrontal cortex (Blair, 2004). In humans, atrophy of the frontal cortex is positively correlated with increases in violent behavior, and aggressive and violent individuals have reduced regional cerebral blood flow and function, primarily in the orbital prefrontal cortex (Bach et al, 1971; Duffy and Campbell, 1994; Raine et al, 1994, 1998; Soderstrom et al, 2000; Soloff et al, 2003). In highly aggressive rats, c-Fos immunoreactivity, a marker of neuronal activity, was significantly elevated in the medial prefrontal and orbitofrontal regions of the prefrontal cortex, again, highlighting the importance of this region in the regulation of aggression (Halász et al, 2006; Haller et al, 2006). Our studies have added to this evidence by showing a pharmacological enhancement of Alc-heightened aggression when CP-94,253 is infused into the mPFC and no effect when infused into either the OFC or the lateral septum, regions immediately anterior and posterior to the mPFC, respectively. This study, along with others, has confirmed that 5-HT1B activation in the prefrontal cortex leads to either increases or decreases in aggressive behavior depending on both the animal model and subregion of the cortex that is targeted (de Almeida et al, 2006). Interestingly, these findings are in accordance with the demonstration that 5-HT1B mRNA in the prefrontal cortex is significantly reduced in mice that have been characterized as Alc-heightened aggressors (Chiavegatto et al, 2007). A reduction in expression of the 5-HT1B receptor, in the targeted region of the microinjection, is a plausible explanation for why Alc-naïve (de Almeida et al, 2006) and Alc-heightened aggressive mice (experiment 2) exhibit such profoundly different behavioral responses to intra-cortical CP-94,253 microinjections.
Initially, the aggression-heightening effect of the 5-HT1B agonist, CP-94,253, was surprising because it does not reflect the prevailing findings proposing an anti-aggressive role for this receptor (De Almeida et al, 2001, 2006; Fish et al, 1999; Grimes and Melloni, 2005; Olivier and Van Oorschot, 2005; Bannai et al, 2007; Veiga et al, 2007). However, in addition to reductions in 5-HT1B mRNA, Chiavegatto et al (2007) also found decreases in prefrontal mRNA transcript for 5-HT1A, 5-HT2A, 5-HT2C, 5-HT6, and 5-HT7 receptors. Since CP-94,253 potentiated Alc-heightened aggression only at the highest dose tested (1 μg), we speculate that the selectivity for 5-HT1B receptors might be diminished, particularly in light of reduced expression; activity at lower-affinity receptors may contribute to this effect (Koe et al, 1992b). The affinity of CP-94,253 at 5-HT6 and 5-HT7 receptors is not known and its relative affinity for the 5-HT1D receptor is presumably heightened, given the reduced expression of the 5-HT1A and 5-HT1B receptors pointing to these receptors as possible mechanisms of action for the aggression-heightening effects of this ligand.
Species-typical aggression was not attenuated after CP-94,253 injection into the mPFC and LS; this result is in accordance with the findings of de Almeida et al (2006), who injected this 5-HT1B agonist into the mPFC of Alc-naïve mice prior to an aggressive encounter. However, they found a significant reduction in aggressive behavior when CP-94,253 was infused into the OFC at the same dose that produced no effect in experiment 3. Differential results between these studies are most likely the result of variations in experimental history of the subjects (naïve vs chronic Alc drinkers) coupled with differences in the constitutive levels of 5-HT1B receptors in these regions (Chiavegatto et al, 2007). Together, these studies suggest that the divergent effect of CP-94,253 in the mPFC vs the OFC indicates that the mPFC is functionally distinct in its modulation of aggressive behavior.
The microdialysis findings further support the hypothesis that there is a significant interaction between medial prefrontal activity of CP-94,253 and Alc drinking. In naïve mice, local application of CP-94,253 significantly increases extracellular levels of 5-HT; this effect was not only antagonized but was reversed in mice with a history of Alc drinking. These results generate several interesting hypotheses. First, it has been demonstrated repeatedly that systemic injection of CP-94,253 decreases extracellular levels of 5-HT in the prefrontal cortex, striatum, and hippocampus (Knobelman et al, 2000; Johnson et al, 2001; De Groote et al, 2003; Miczek et al, 2004a). Because this attenuation was not observed in the mPFC during local perfusion of the same ligand, it is likely that the decreased 5-HT seen in these terminal regions after systemic administration is due to a reduction in 5-HT cell firing and release following somatodendritic receptor stimulation rather than to activity at pre- or post-synaptic terminal receptors. Second, the profound difference in the effect of CP-94,253 on prefrontal 5-HT levels in a naïve vs chronic Alc-drinking mouse suggests that even moderate Alc drinking is altering the serotonergic ‘tone’ of the Alc drinker, which may be reflected, in the present experiment, as a different neurochemical response to a pharmacological challenge. Chronic Alc self-administration leads to long-lasting neuroadaptive changes in multiple neurotransmitter systems and in vivo, ethanol reduces the persistent activity of prefrontal cortical neurons (McBride and Li, 1998; Tu et al, 2007). Some studies have suggested that the 5-HT1B receptor is involved in regulating Alc self-administration and preference (Crabbe et al, 1996; Hoplight et al, 2006). The currently studied mice self-administered moderate doses of Alc for a minimum of 2 months prior to the microinjection experiments. Differences in basal levels of a neurotransmitter are ideally quantified using a no-net-flux method that calculates a standard curve for the amount of neurotransmitter in a given region by perfusing several known concentration through the microdialysis probe and the amount retained in the dialysate (Parsons and Justice, 1994). The use of this method in this experiment was not feasible due to limitations in sample collection and the high degree of difficulty in analyzing small samples from a mouse mPFC but it is acknowledged that these are important future experiments.
At high concentrations, CP-94,253 may be recruiting post-synaptic receptors, which would account for the opposite behavioral response seen in the DRN vs mPFC. Inactivation of somatodendritic 5-HT1B receptors by intra-raphé 5,7-DHT or PCPA injections fails to attenuate the antiaggressive- and antidepressant-like effects of 5-HT1B agonists, suggesting that these behaviors are modulated by post-synaptic 5-HT1B heteroreceptor activity (Clark and Neumaier, 2001; de Almeida et al, 2001; Tatarczyñska et al, 2005). Local administration of 5-HT1B agonists decreases extracellular levels of glutamate in the prefrontal cortex, which, presumably, is due to activation of 5-HT1B receptors located on cortical glutamatergic neurons (Gołembiowska and Dziubina, 2002). Mounting evidence implicates a role for mPFC glutamate in the modulation of impulsive behavior and cognitive impairments (Goff and Coyle, 2001). Specifically, pharmacological antagonism of NMDA receptors in the prefrontal cortex or prefrontal legions have been shown to increase perseverative and anticipatory responding in rodent models of impulsive choice (Murphy et al, 2005; Carli et al, 2006; Baviera et al, 2007). Alc, being a glutamatergic antagonist, might act in a similar way to produce prefrontal behavioral impairments and to interact with the decrease in cortical excitatory neurotransmission induced by CP-94,253, which could functionally result in increased impulsivity and aggression (Lovinger et al, 1989).
In conclusion, one of the most consistent findings in aggression research is that 5-HT agonists decrease aggressive behavior in multiple species, under various conditions involving receptors in the 5-HT1 and 5-HT2 families (Olivier and Mos, 1986; Sanchez et al, 1993; Muehlenkamp et al, 1995; Miczek et al, 1998, 2007; Fish et al, 1999; de Almeida et al, 2001). The heightened aggressive behavior that we have shown after medial prefrontal microinjection of CP-94,253 is intriguing because it (1) further confirms that the 5-HT1B receptor is one of the few receptors that specifically modulates aggressive behavior and (2) reveals that selective increases in aggressive behavior are possible via a serotonergic mechanism. Further study of these neurochemical mechanisms that regulate Alc-heightened aggression is important because they facilitate understanding of one facet of Alc abuse—behavioral disruptions and impaired impulse control.
References
Adell A, Carceller A, Artigas F (1993). In vivo brain dialysis study of the somatodendritic release of serotonin in the raphe nuclei of the rat: Effects of 8-Hydroxy-2-(Di-{In}- Propylamino)tetralin. J Neurochem 60: 1673–1681.
Adell A, Celada P, Artigas F (2001). The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem 79: 172–182.
Bach YR, Lion JR, Climent CE, Ervin FR (1971). Episodic dyscontrol: A study of 130 violent patients. Am J Psychiatry 127: 1473–1478.
Bannai M, Fish EW, Faccidomo S, Miczek KA (2007). Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 193: 295–304.
Baviera M, Invernizzi RW, Carli M (2007). Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berl) 196: 269–280.
Bechara A, Tranel D, Damasio H (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123 (Part 11): 2189–2202.
Best M, Williams JM, Coccaro EF (2002). Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci USA 99: 8448–8453.
Blair RJR (2004). The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn 55: 198–208.
Brower MC, Price BH (2001). Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. J Neurol Neurosurg Psychiatry 71: 720–726.
Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA et al (1994). Localization of 5-HT1B, 5-HT1D α, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33: 367–386.
Bruinvels AT, Palacios JM, Hoyer D (1993). Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol 347: 569–582.
Carli M, Baviera M, Invernizzi RW, Balducci C (2006). Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology 31: 757–767.
Chance MRA, Mackintosh JH, Dixon AK (1973). The effects of ethyl alcohol on social encounters between mice. J Alcoholism 8: 90–93.
Chiavegatto S, Quadros IMH, Trindade A, Ambar G, Miczek KA (2007). Alcohol-heightened aggression in mice is associated with reduced mRNA levels of serotonin receptors in the prefrontal cortex. Alcohol Clin Exp Res 31: 190A.
Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146: 105–119.
Clark MS, Neumaier JF (2001). The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull 35: 170–185.
Coccaro EF (1992). Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain–behavior relationship. Int Clin Psychopharmacol 7: 3–12.
Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN et al (1996). Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet 14: 98–101.
de Almeida RM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA (2006). 5-HT1B receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology (Berl) 185: 441–450.
de Almeida RMM, Lucion AB (1997). 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology (Berl) 134: 392–400.
de Almeida RMM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA (2001). Zolmitriptan—a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology (Berl) 157: 131–141.
de Boer SF, Koolhaas JM (2005). 5-HT1A and 5-HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526: 125–139.
De Groote L, Olivier B, Westenberg HG (2003). Role of 5-HT1B receptors in the regulation of extracellular serotonin and dopamine in the dorsal striatum of mice. Eur J Pharmacol 476: 71–77.
Duffy JD, Campbell JJ (1994). The regional prefrontal syndromes—a theoretical and clinical overview. J Neuropsychiatry Clin Neurosci 6: 379–387.
Ferrari PF, van Erp AMM, Tornatzky W, Miczek KA (2003). Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci 17: 371–378.
Fish EW, Faccidomo S, Miczek KA (1999). Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology (Berl) 146: 391–399.
Fish EW, McKenzie-Quirk SD, Bannai M, Miczek KA (2007). 5-HT1B receptor inhibition of alcohol-heightened aggression in mice: comparison to drinking and running. Psychopharmacology (Berl) (E-pub 2007 Dec 11).
Garattini S, Giacalone E, Valzelli L (1967). Isolation, aggressiveness and brain 5-hydroxytryptamine turnover. J Pharm Pharmacol 19: 338–339.
Giacalone E, Tansella M, Valzelli L, Garattini S (1968). Brain serotonin metabolism in isolated aggressive mice. Biochem Pharmacol 17: 1315–1327.
Goff DC, Coyle JT (2001). The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158: 1367–1377.
Gołembiowska K, Dziubina A (2002). Inhibition of amino acid release by 5-HT1B receptor agonist in the rat prefrontal cortex. Pol J Pharmacol 54: 625–631.
Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM (1996). Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology 46: 1231–1238.
Grant EC, Mackintosh JH (1963). A comparison of the social postures of some common laboratory rodents. Behaviour 21: 246–295.
Grimes JM, Melloni Jr RH. (2005). Serotonin-1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behav Neurosci 119: 1184–1194.
Halász J, Tóth M, Kalló I, Liposits Z, Haller J (2006). The activation of prefrontal cortical neurons in aggression—a double labeling study. Behav Brain Res 175: 166–175.
Haller J, Tóth M, Halász J, DeBoer SF (2006). Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol Behav 88: 173–182.
Higley JD, Mehlman PT, Higley SB, Fernald B, Vickers J, Lindell SG et al (1996). Excessive mortality in young free-ranging male nonhuman primates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Arch Gen Psychiatry 53: 537–543.
Hoplight BJ, Sandygren NA, Neumaier JF (2006). Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol 38: 73–79.
Hoyer D, Engel G, Kalkman HO (1985). Characterization of the 5-HT1B recognition site in rat brain: Binding studies with (–)[125I]iodocyanopindolol. Eur J Pharmacol 118: 1–12.
Johnson DE, Rollema H, Schmidt AW, McHarg AD (2001). Serotonergic effects and extracellular brain levels of eletriptan, zolmitriptan and sumatriptan in rat brain. Eur J Pharmacol 425: 203–210.
Knobelman DA, Kung HF, Lucki I (2000). Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT1A and 5-HT1B receptors. J Pharmacol Exp Ther 292: 1111–1117.
Koe BK, Lebel LA, Fox CB, Macor JE (1992a). Binding and uptake studies with [H-3] CP-93,129, a radiolabeled selective 5-HT1B receptor ligand. Drug Dev Res 25: 67–74.
Koe BK, Nielsen JA, Macor JE, Heym J (1992b). Biochemical and behavioral studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res 26: 241–250.
Lister RG, Hilakivi LA (1988). The effects of novelty, isolation, light and ethanol on the social behavior of mice. Psychopharmacology (Berl) 96: 181–187.
Lovinger DM, White G, Weight FF (1989). Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724.
McBride WJ, Li TK (1998). Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12: 339–369.
Mehlman Pt, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J et al (1994). Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry 151: 1485–1491.
Miczek KA, de Almeida RMM (2001). Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology (Berl) 157: 421–429.
Miczek KA, Faccidomo S, de Almeida RMM, Bannai M, Fish EW, DeBold JF (2004a). Escalated aggressive behavior: pharmacotherapeutic approaches and opportunities. Ann NY Acad Sci 1036: 336–355.
Miczek KA, Faccidomo SP, Fish EW, DeBold JF (2007). Neurochemistry and molecular neurobiology of aggressive behavior. In: JD Blaustein (ed). Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. Handbook of Neurochemistry and Molecular Neurobiology. Springer: New York, pp 286–316.
Miczek KA, Fish EW (2006). Monoamines, GABA, Glutamate, and Aggression. In: Nelson RJ (ed). Biology of Aggression. Oxford University Press: New York, pp 114–149.
Miczek KA, Fish EW, de Almeida RMM, Faccidomo S, DeBold JF (2004b). Role of alcohol consumption in escalations to violence. Ann NY Acad Sci 1036: 278–289.
Miczek KA, Hussain S, Faccidomo S (1998). Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology (Berl) 139: 160–168.
Miczek KA, O'Donnell JM (1978). Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology 57: 47–55.
Miczek KA, O'Donnell JM (1980). Alcohol and chlordiazepoxide increase suppressed aggression in mice. Psychopharmacology (Berl) 69: 39–44.
Miczek KA, Weerts EM, DeBold JF (1993). Alcohol, benzodiazepine-GABAA receptor complex and aggression: ethological analysis of individual differences in rodents and primates. J Stud Alcohol suppl 11: 170–179.
Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H (1993). The effects of dorsal raphe administration of eltoprazine, TFMPP and 8-OH- DPAT on resident intruder aggression in the rat. Eur J Pharmacol 238: 411–415.
Muehlenkamp F, Lucion A, Vogel WH (1995). Effects of selective serotenergic agonists on aggressive behavior in rats. Pharmacol Biochem Behav 50: 671–674.
Murphy ER, Dalley JW, Robbins TR (2005). Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 179: 99–107.
National Research Council (1996). Guide for the Care and Use of Laboratory Animals. National Academy Press: Washington DC.
Olivier B, Mos J (1986). Serenics and aggression. Stress Med 2: 197–209.
Olivier B, Mos J (1992). Rodent models of aggressive behavior and serotonergic drugs. Prog Neuropsychopharmacol Biol Psychiatry 16: 847–870.
Olivier B, Mos J, Van der Heyden J, Hartog J (1989). Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology (Berl) 97: 154–156.
Olivier B, Mos J, Van Oorschot R, Hen R (1995). Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 28: 80–90.
Olivier B, Van Oorschot R (2005). 5-HT1B receptors and aggression: A review. Eur J Pharmacol 526: 207–217.
Parsons LH, Justice Jr JB. (1994). Quantitative approaches to in vivo brain microdialysis. Crit Rev Neurobiol 8: 189–220.
Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates Second Edition Academic Press: San Diego.
Peeke HVS, Ellman GE, Herz MJ (1973). Dose dependent alcohol effects on the aggressive behavior of the conflict cichlid (Cichlasoma nigrofaciatum). Behav Biol 8: 115–122.
Perez M, Pauwels PJ, Fourrier C, Chopin P, Valentin JP, John GW et al (1998). Dimerization of sumatriptan as an efficient way to design a potent, centrally and orally active 5-HT1B agonist. Bioorg Med Chem Lett 8: 675–680.
Pineyro G, Blier P (1999). Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev 51: 533–591.
Raine A, Buchsbaum MS, Stanley J, Lottenberg S, Abel L, Stoddard J (1994). Selective reductions in prefrontal glucose metabolism in murderers. Biol Psychiatry 36: 365–373.
Raine A, Meloy JR, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS (1998). Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law 16: 319–332.
Sanchez C, Arnt J, Hyttel J, Moltzen EK (1993). The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology (Berl) 110: 53–59.
Sari Y (2004). Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28: 565–582.
Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M et al (1999). Cellular and subcellular localization of 5-Hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88: 899–915.
Soderstrom H, Tullberg M, Wikkelso C, Ekholm S, Forsman A (2000). Reduced regional cerebral blood flow in non-psychotic violent offenders. Psychiatry Res 98: 29–41.
Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D (2003). Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Res 123: 153–163.
Spinella M (2004). Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int J Neurosci 114: 95–104.
Tatarczyñska E, Antkiewicz-Michaluk L, Kłodziñska A, Stachowicz K, Chojnacka-Wójcik E (2005). Antidepressant-like effect of the selective 5-HT1B receptor agonist CP 94253: a possible mechanism of action. Eur J Pharmacol 516: 46–50.
Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ et al (2007). Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci 27: 4765–4775.
Van Der Vegt BJ, de Boer SF, Buwalda B, de Ruiter AJ, de Jong JG, Koolhaas JM (2001). Enhanced sensitivity of postsynaptic serotonin-1A receptors in rats and mice with high trait aggression. Physiol Behav 74: 205–211.
van Erp AMM, Miczek KA (2000). Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci 20: 9320–9325.
Veiga CP, Miczek KA, Lucion AB, Almeida RM (2007). Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res 40: 825–830.
Virkkunen M, Linnoila M (1993). Brain serotonin, Type II alcoholism and impulsive violence. J Stud Alcohol 11: 163–169.
Acknowledgements
We would like to thank J Thomas Sopko for his outstanding technical assistance, Rachel vanTrigt and Jeremy Maggin for their assistance in conducting the self-administration and aggression experiments, Satoko Bannai for her tremendous help with the HPLC, and Dr Eric W Fish for consultation and advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that this research was supported by an NIAAA grant to KAM (AA013983) and by Ajinomoto Co. Inc. (MB). The authors declare that except for income received by their primary employers, they have not received any financial support or compensation that could be perceived as a conflict of interest.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary information
Rights and permissions
About this article
Cite this article
Faccidomo, S., Bannai, M. & Miczek, K. Escalated Aggression after Alcohol Drinking in Male Mice: Dorsal Raphé and Prefrontal Cortex Serotonin and 5-HT1B Receptors. Neuropsychopharmacol 33, 2888–2899 (2008). https://doi.org/10.1038/npp.2008.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2008.7
Keywords
This article is cited by
-
Effects of Acute Stress in Mice with 5-HT1A Receptors with Different Sensitivities to Chronic Activation by 8-OH-DPAT
Neuroscience and Behavioral Physiology (2021)
-
Ethanol facilitates socially evoked memory recall in mice by recruiting pain-sensitive anterior cingulate cortical neurons
Nature Communications (2018)
-
The 5-HT1B receptor - a potential target for antidepressant treatment
Psychopharmacology (2018)
-
EphB2 in the Medial Prefrontal Cortex Regulates Vulnerability to Stress
Neuropsychopharmacology (2016)
-
Ethanol Increases Mechanical Pain Sensitivity in Rats via Activation of GABAA Receptors in Medial Prefrontal Cortex
Neuroscience Bulletin (2016)