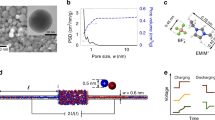
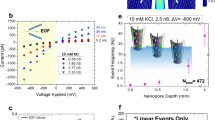
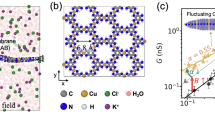
Abstract
Nanoscale pores exhibit transport properties that are not seen in micrometre-scale pores, such as increased ionic concentrations inside the pore relative to the bulk solution, ionic selectivity and ionic rectification. These nanoscale effects are all caused by the presence of permanent surface charges on the walls of the pore. Here we report a new phenomenon in which the addition of small amounts of divalent cations to a buffered monovalent ionic solution results in an oscillating ionic current through a conical nanopore. This behaviour is caused by the transient formation and redissolution of nanoprecipitates, which temporarily block the ionic current through the pore. The frequency and character of ionic current instabilities are regulated by the potential across the membrane and the chemistry of the precipitate. We discuss how oscillating nanopores could be used as model systems for studying nonlinear electrochemical processes and the early stages of crystallization in sub-femtolitre volumes. Such nanopore systems might also form the basis for a stochastic sensor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ashcroft, F. M. Ion Channels and Disease (Academic Press, New York, 1999).
Eisenberg, R. S. Atomic biology, electrostatics and ionic channels. In New Developments and Theoretical Studies of Proteins Vol. 7 (ed. Elber, R.) Ch. 5, 269–357, Advanced Series in Physical Chemistry (World Scientific, Philadelphia, 1996).
Eisenberg, R. S. Ionic channels as natural nanodevices. J. Comp. Electr. 1, 331–334 (2002).
Kasianowicz, J. J., Brandin, E., Branton, D. & Deamer, D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl Acad. Sci. USA 93, 13770–13773 (1996).
Bayley, H. & Martin, C. R. Resistive-pulse sensing—from microbes to molecules. Chem. Rev. 100, 2575–2594 (2000).
Dekker, C. Solid-state nanopores. Nature Nanotech. 2, 209–215 (2007).
Uram, J. D., Ke, K., Hunt, A. J. & Mayer, M. Submicrometer pore-based characterization and quantification of antibody–virus interactions. Small 2, 967–972 (2006).
Iqbal, S. M., Akin, D. & Bashir, R. Solid-state nanopore channels with DNA selectivity. Nature Nanotech. 2, 243–248 (2007).
Berezhkovskii, A. M., Hummer, G. & Bezrukov, S. M. Identity of distributions of direct uphill and downhill translocation times for particles traversing membrane channels. Phys. Rev. Lett. 97, 020601 (2006).
Muthukumar, M. Polymer escape through a nanopore. J. Chem. Phys. 118, 5174–5184 (2003).
Keyser, U. F. et al. Direct force measurements on DNA in a solid-state nanopore. Nature Phys. 2, 473–477 (2006).
Stein, D., Kruithof, M. & Dekker, C. Surface-charge-governed ion transport in nanofluidic channels. Phys. Rev. Lett. 93, 035901 (2004).
Israelachvili, J. Intermolecular and Surface Forces 2nd edn (Academic Press, London, 1991).
Daiguji, H., Yang, P. & Majumdar, A. Ion transport in nanofluidic channels. Nano Lett. 4, 137–142 (2004).
Vlassiouk, I. & Siwy, Z. Nanofluidic diode. Nano Lett. 7, 552–556 (2007).
Karnik, R., Duan, C., Castelino, K., Daiguji, H. & Majumdar, A. Rectification of ionic current in a nanofluidic diode. Nano Lett. 7, 547–551 (2007).
Karnik, R. et al. Electrostatic control of ions and molecules in nanofluidic transistors. Nano Lett. 5, 943–948 (2005).
Eisenberg, R. S. Computing the field in proteins and channels. J. Membrane Biol. 150, 1–25 (1996).
Fleischer, R. L., Price, P. B. & Walker, R. M. Nuclear Tracks in Solids. Principles and Applications (Univ. of California Press, Berkeley, 1975).
Apel A., Korchev Y. E., Siwy Z., Spohr R. & Yoshida M. Diode-like single-ion track membrane prepared by electro-stopping. Nucl. Instrum. Methods Phys. Res. B 184, 337–346 (2001).
Siwy, Z., Powell, M. R., Kalman, E., Astumian, R. D. & Eisenberg, R. S. Negative incremental resistance induced by calcium in asymmetric nanopores. Nano Lett. 6, 473–477 (2006).
Siwy, Z. et al. Calcium-induced voltage gating in single conical nanopores. Nano Lett. 6, 1729–1734 (2006).
Dean, J. A. Lange's Handbook of Chemistry 15th edn (McGraw-Hill, New York, 1999).
Cervera, J., Schiedt, B. & Ramirez, P. A Poisson/Nernst–Planck model for ionic transport through synthetic conical nanopores. Europhys. Lett. 71, 35–41 (2005).
Siwy Z. & Fulinski A. Fabrication of a synthetic nanopore ion-pump. Phys. Rev. Lett. 89, 198103 (2002).
Ciavatti, L. The Specific Interaction Theory in equilibrium analysis. Some empirical rules for estimating interaction coefficients of metal ion complexes. Anali di Chimica by Societa Chimica Italiana 80, 255–263 (1990).
Ciavatti, L. The Specific Interaction Theory in evaluating ionic equilibria. Anali di Chimica by Societa Chimica Italiana 80, 551–567 (1980)
Nonner, W. & Eisenberg, R. S. Ion permeation and glutamate residues linked by Poisson–Nernst–Planck theory in L-type calcium channels. Biophys. J. 75, 1287–1305 (1998).
Constantin, D. & Siwy, Z. Poisson–Nernst–Planck model of ion current rectification through a nanofluidic diode. Phys. Rev. E 76, 041202 (2007).
Krischer, K., Mazouz, N. & Grauel, P. Fronts, waves, and stationary patterns in electrochemical systems. Angew. Chem. Int. Edn 40, 850–859 (2001).
Kurin-Csörgei, K., Epstein, I. R. & Orban, M. Systematic design of chemical oscillators using complexation and precipitation equilibria. Nature 433, 139–142 (2005).
Epstein I. R. & Pojman, J. A. Introduction to Nonlinear Chemical Dynamics. Oscillations, Waves, Patterns and Chaos (Oxford Univ. Press, New York, 1998).
Bayley, H. & Cremer, P. S. Stochastic sensors inspired by biology. Nature 413, 226–230 (2001).
Brindley, G. W. & Kao, C-C. Structural and IR relations among brucite-like divalent metal hydroxides. Phys. Chem. Minerals 10, 187–191 (1984).
Dickens, B., Bowen, J. S. & Brown, W. E. A refinement of the crystal structure of CaHPO4 (synthetic monetite). Acta Cryst. B28, 797–806 (1971).
Krishna, R. & Wesselingh, J. A. The Maxwell–Stefan approach to mass transfer. Chem. Eng. Sci. 52, 861–911 (1997).
Acknowledgements
Irradiation with swift heavy ions was performed at the Gesellschaft für Schwerionenforschung (GSI), Darmstadt, Germany. We thank the Alfred P. Sloan Foundation, the IM-SURE undergraduate programme, the Institute for Surface and Interface Science and the Institute for Complex Adaptive Matter for financial support.
Author information
Authors and Affiliations
Contributions
Z.S., R.S.E. and I.V. conceived the experiments. M.R.P., M.S. and I.V. performed the experiments. D.C. analysed the data and was in charge of calculations. M.R.P. and Z.S. wrote the manuscript. R.S. co-wrote the manuscript. O.S. and C.C.M. analysed the data, discussed the results, explained the transient character of precipitation formation, and co-wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1–S12 (PDF 718 kb)
Rights and permissions
About this article
Cite this article
Powell, M., Sullivan, M., Vlassiouk, I. et al. Nanoprecipitation-assisted ion current oscillations. Nature Nanotech 3, 51–57 (2008). https://doi.org/10.1038/nnano.2007.420
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2007.420
This article is cited by
-
Transportation of calcium ions through chemically modified nanochannels in a polymeric membrane
Ionics (2022)
-
Smart polymer-based calcium-ion self-regulated nanochannels by mimicking the biological Ca2+-induced Ca2+ release process
NPG Asia Materials (2019)
-
Ionic switch using nano-channels in polymeric membrane
Ionics (2019)
-
Ion current rectification: from nanoscale to microscale
Science China Chemistry (2019)
-
Redox switch of ionic transport in conductive polypyrrole-engineered unipolar nanofluidic diodes
Nano Research (2017)