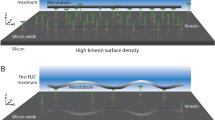
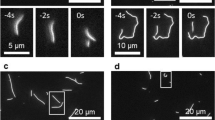
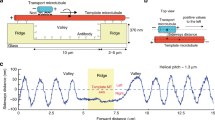
Abstract
Wear is the progressive loss of material from a body caused by contact and relative movement and is a major concern in both engineering and biology1,2,3,4. Advances in nanotechnology have allowed the origins of wear processes to be studied at the atomic and molecular scale, but also demand that wear in nanoscale systems can be predicted and controlled5,6,7. Biomolecular systems can undergo a range of active movements at the nanoscale, which are enabled by the transduction of chemical energy into mechanical work by polymerization processes and motor proteins8. The active movements are accompanied by dissipative processes that can be conceptually understood as ‘protein friction’9,10. Here, we show that wear also occurs in an in vitro system consisting of microtubules gliding across a surface coated with kinesin-1 motor proteins, and that energetic considerations suggest a molecule-by-molecule removal of tubulin proteins. The rates of removal show a complex dependence on sliding velocity and kinesin density, which, in contrast to the friction behaviour between microtubules and kinesin-8, cannot be explained by simple chemical reaction kinetics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ludema, K. C. Friction, Wear, Lubrication: A Textbook in Tribology (CRC Press, 1996).
Norton, R. L. Machine Design: An Integrated Approach (Prentice Hall, 2011).
Hutchings, I. M. Tribology: Friction and Wear of Engineering Materials (CRC Press, 1992).
Erickson, G. M. et al. Complex dental structure and wear biomechanics in hadrosaurid dinosaurs. Science 338, 98–101 (2012).
Bhushan, B. Nanotribology and nanomechanics in nano/biotechnology. Phil. Trans. R. Soc. Lond. A 366, 1499–1537 (2008).
Gao, G. T., Mikulski, P. T. & Harrison, J. A. Molecular-scale tribology of amorphous carbon coatings: effects of film thickness, adhesion, and long-range interactions. J. Am. Chem. Soc. 124, 7202–7209 (2002).
Jacobs, T. D. B. & Carpick, R. W. Nanoscale wear as a stress-assisted chemical reaction. Nature Nanotech. 8, 108–112 (2013).
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton (Sinauer, 2001).
Bormuth, V., Varga, V., Howard, J. & Schaffer, E. Protein friction limits diffusive and directed movements of kinesin motors on microtubules. Science 325, 870–873 (2009).
Tawada, K. & Sekimoto, K. Protein friction exerted by motor enzymes through a weak-binding interaction. J. Theor. Biol. 150, 193–200 (1991).
Varga, V., Leduc, C., Bormuth, V., Diez, S. & Howard, J. Kinesin-8 motors act cooperatively to mediate length-dependent microtubule depolymerization. Cell 138, 1174–1183 (2009).
Wilson, L. et al. Taxol stabilization of microtubules in vitro—dynamics of tubulin addition and loss at opposite microtubule ends. Biochemistry 24, 5254–5262 (1985).
Schiff, P. B., Fant, J. & Horwitz, S. B. Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667 (1979).
Nitta, T. & Hess, H. Dispersion in active transport by kinesin-powered molecular shuttles. Nano Lett. 5, 1337–1342 (2005).
Brunner, C., Hess, H., Ernst, K-H. & Vogel, V. Lifetime of biomolecules in hybrid nanodevices. Nanotechnology 15, S540–S548 (2004).
Ruhnow, F., Zwicker, D. & Diez, S. Tracking single particles and elongated filaments with nanometer precision. Biophys. J. 100, 2820–2828 (2011).
Vale, R. D., Schnapp, B. J., Reese, T. S. & Sheetz, M. P. Organelle, bead, and microtubule translocations promoted by soluble factors from the squid giant axon. Cell 40, 559–569 (1985).
Diaz-Valencia, J. D. et al. Drosophila katanin-60 depolymerizes and severs at microtubule defects. Biophys. J. 100, 2440–2449 (2011).
Howard, J. & Hyman, A. A. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence microscopy. Methods Cell Biol. 39, 105–113 (1993).
Aggarwal, T., Materassi, D., Davison, R., Hays, T. & Salapaka, M. Detection of steps in single molecule data. Cell. Mol. Bioeng. 5, 14–31 (2012).
Dumont, E. L. P., Belmas, H. & Hess, H. Observing the mushroom-to-brush transition for kinesin proteins. Langmuir 29, 15142–15145 (2013).
Toprak, E., Yildiz, A., Hoffman, M. T., Rosenfeld, S. S. & Selvin, P. R. Why kinesin is so processive. Proc. Natl Acad. Sci. USA 106, 12717–12722 (2009).
VanBuren, V., Cassimeris, L. & Odde, D. J. Mechanochemical model of microtubule structure and self-assembly kinetics. Biophys. J. 89, 2911–2926 (2005).
Agarwal, A., Katira, P. & Hess, H. Millisecond curing time of a molecular adhesive causes velocity-dependent cargo-loading of molecular shuttles. Nano Lett. 9, 1170–1175 (2009).
Neuert, G., Albrecht, C. H. & Gaub, H. E. Predicting the rupture probabilities of molecular bonds in series. Biophys. J. 93, 1215–1223 (2007).
Bhaskaran, H. et al. Ultralow nanoscale wear through atom-by-atom attrition in silicon-containing diamond-like carbon. Nature Nanotech. 5, 181–185 (2010).
Gnecco, E., Bennewitz, R. & Meyer, E. Abrasive wear on the atomic scale. Phys. Rev. Lett. 88, 215501 (2002).
Gotsmann, B. & Lantz, M. A. Atomistic wear in a single asperity sliding contact. Phys. Rev. Lett. 101, 125501 (2008).
Coy, D. L., Hancock, W. O., Wagenbach, M. & Howard, J. Kinesin's tail domain is an inhibitory regulator of the motor domain. Nature Cell Biol. 1, 288–292 (1999).
Howard, J., Hunt, A. J. & Baek, S. Assay of microtubule movement driven by single kinesin molecules. Methods Cell. Biol. 39, 137–147 (1993).
Wettermark, G., Borglund, E. & Brolin, S. E. A regenerating system for studies of phosphoryl transfer from ATP. Anal. Biochem. 22, 211–218 (1968).
Acknowledgements
The authors acknowledge financial support from the National Science Foundation (grant CMMI-0926780) and from a Liu Ping fellowship (E.L.P.D.) and thank G. Bachand for providing the kinesin protein. This work was performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Los Alamos National Laboratory (contract DE-AC52-06NA25396) and Sandia National Laboratories (contract DE-AC04-94AL85000).
Author information
Authors and Affiliations
Contributions
E.L.P.D. and H.H. designed and conceived the experiments. E.L.P.D. performed the experiments. E.L.P.D. and C.D. performed the statistical analysis. E.L.P.D. and H.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Information (PDF 2878 kb)
Rights and permissions
About this article
Cite this article
Dumont, E., Do, C. & Hess, H. Molecular wear of microtubules propelled by surface-adhered kinesins. Nature Nanotech 10, 166–169 (2015). https://doi.org/10.1038/nnano.2014.334
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2014.334
This article is cited by
-
The rate of microtubule breaking increases exponentially with curvature
Scientific Reports (2022)
-
Self-repair protects microtubules from destruction by molecular motors
Nature Materials (2021)
-
Synchronous operation of biomolecular engines
Biophysical Reviews (2020)
-
The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology
Neural Development (2019)
-
Kinesin motor density and dynamics in gliding microtubule motility
Scientific Reports (2019)