Abstract
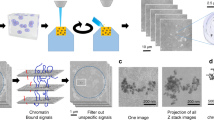
The difficulty in localizing specific cellular proteins by immuno-electron microscopy techniques limits applications of electron microscopy to cell biology. We found that in vivo immunogold labeling improves epitope accessibility, ultrastructural preservation and three-dimensional visualization, and allows correlated light and electron microscopy. We detected large-scale chromatin folding motifs within intact interphase nuclei of CHO cells and visualized the ultrastructure of DNA replication 'factories' labeled with GFP–proliferating cell nuclear antigen (PCNA).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gaietta, G. et al. Science 296, 503–507 (2002).
Grabenbauer, M. et al. Nat. Methods 2, 857–862 (2005).
Li, G., Sudlow, G. & Belmont, A.S. J. Cell Biol. 140, 975–989 (1998).
Hainfeld, J.F. & Furuya, F.R. J. Histochem. Cytochem. 40, 177–184 (1992).
Biggiogera, M. et al. Biol. Cell 87, 121–132 (1996).
Testillano, P.S. et al. J. Histochem. Cytochem. 39, 1427–1438 (1991).
Derenzini, M., Farabegoli, F. & Trere, D. J. Histochem. Cytochem. 41, 829–836 (1993).
Belmont, A.S., Braunfeld, M.B., Sedat, J.W. & Agard, D.A. Chromosoma 98, 129–143 (1989).
Robinett, C.C. et al. J. Cell Biol. 135, 1685–1700 (1996).
Kireeva, N., Lakonishok, M., Kireev, I., Hirano, T. & Belmont, A.S. J. Cell Biol. 166, 775–785 (2004).
Leonhardt, H. et al. J. Cell Biol. 149, 271–280 (2000).
Ma, H. et al. J. Cell Biol. 143, 1415–1425 (1998).
O'Keefe, R.T., Henderson, S.C. & Spector, D.L. J. Cell Biol. 116, 1095–1110 (1992).
Jaunin, F., Visser, A.E., Cmarko, D., Aten, J.A. & Fakan, S. Exp. Cell Res. 260, 313–323 (2000).
Hozak, P., Hassan, A.B., Jackson, D.A. & Cook, P.R. Cell 73, 361–373 (1993).
Acknowledgements
This work was supported by the US National Institute of General Medical Sciences. (grant GM42516 to A.S.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
I.K. performed in vivo labeling and electron microscopy analysis; M.L. developed the monoclonal antibodies, purified these antibodies and prepared Fab′ fragments, and carried out initial in vivo labeling feasibility experiments; R.P., W.L. and V.N.J. optimized and conducted the Nanogold antibody labeling, in conjunction with M.L.; A.S.B. supervised the overall project.
Corresponding author
Ethics declarations
Competing interests
W.L., V.N.J. and R.P. are employees of Nanoprobes, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Methods (PDF 1764 kb)
Supplementary Movie 1
Tilted projections showing gene amplified region from PDC cell labeled in vivo with anti-GFP Nanogold antibodies. Large-scale chromatin fibers of 120-170 nm diameter can be visualized corresponding to the GFP-lac repressor staining of the lac operator containing, amplified transgene array. Projection step size 5 degrees. Scale bar, 500 nm. (MOV 1820 kb)
Supplementary Movie 2
Rocking projections of a mid-S phase, DNA replication focus, as identified by GFP-PCNA labeled in vivo with anti-GFP Nanogold antibodies. Note the appearance of a uniform distribution of label through the replication focus. Projection step size 5 degrees. Cell was permeabilized prior to fixation to reveal surrounding chromatin (darkly stained material). (MOV 1281 kb)
Rights and permissions
About this article
Cite this article
Kireev, I., Lakonishok, M., Liu, W. et al. In vivo immunogold labeling confirms large-scale chromatin folding motifs. Nat Methods 5, 311–313 (2008). https://doi.org/10.1038/nmeth.1196
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1196
This article is cited by
-
Effect of Iron Oxide Nanoparticle Shape on Doxorubicin Drug Delivery Toward LNCaP and PC-3 Cell Lines
BioNanoScience (2018)
-
Coming to terms with chromatin structure
Chromosoma (2016)
-
Nanohybride Materials Based on Magnetite-Gold Nanoparticles for Diagnostics of Prostate Cancer: Synthesis and In Vitro Testing
Bulletin of Experimental Biology and Medicine (2016)
-
Constitutive heterochromatin reorganization during somatic cell reprogramming
The EMBO Journal (2011)
-
Analysis of replication factories in human cells by super-resolution light microscopy
BMC Cell Biology (2009)