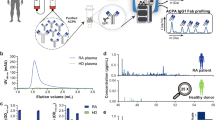
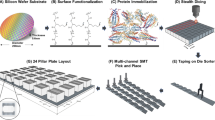
Abstract
Antigen microarrays hold great promise for profiling the humoral immune response in the settings of autoimmunity, allergy and cancer. This approach involves immobilizing antigens on a slide surface and then exposing the array to biological fluids containing immunoglobulins. Although these arrays have proven extremely useful as research tools, they suffer from several sources of variability. To address these issues, we have developed a new two-color Fab labeling method that allows two samples to be applied simultaneously to the same array. This straightforward labeling approach improves reproducibility and reliably detects changes in autoantibody concentrations. Using this technique we profiled serum from a mouse model of systemic lupus erythematosus (SLE) and detected both expected and previously unrecognized reactivities. The improved labeling and detection method described here overcomes several problems that have hindered antigen microarrays and should facilitate translation to the clinical setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lyons, R., Narain, S., Nichols, C., Satoh, M. & Reeves, W.H. Effective use of autoantibody tests in the diagnosis of systemic autoimmune disease. Ann. NY Acad. Sci. 1050, 217–228 (2005).
Robinson, W.H. et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 8, 295–301 (2002).
Robinson, W.H. et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 (2003).
Neuman de Vegvar, H.E. et al. Microarray profiling of antibody responses against simian-human immunodeficiency virus: postchallenge convergence of reactivities independent of host histocompatibility type and vaccine regimen. J. Virol. 77, 11125–11138 (2003).
Hueber, W. et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 52, 2645–2655 (2005).
Li, Q.Z. et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J. Clin. Invest. 115, 3428–3439 (2005).
Wang, X. et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med. 353, 1224–1235 (2005).
Hiller, R. et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 16, 414–416 (2002).
Zhou, H. et al. Two-color, rolling-circle amplification on antibody microarrays for sensitive, multiplexed serum-protein measurements. Genome Biol. 5, R28 (2004).
Hamelinck, D. et al. Optimized normalization for antibody microarrays and application to serum-protein profiling. Mol. Cell. Proteomics 4, 773–784 (2005).
Brown, J.K., Pemberton, A.D., Wright, S.H. & Miller, H.R. Primary antibody-Fab fragment complexes: a flexible alternative to traditional direct and indirect immunolabeling techniques. J. Histochem. Cytochem. 52, 1219–1230 (2004).
Bradford, J.A., Buller, G., Suter, M., Ignatius, M. & Beechem, J.M. Fluorescence-intensity multiplexing: simultaneous seven-marker, two-color immunophenotyping using flow cytometry. Cytometry A 61, 142–152 (2004).
Pusztai, L. & Hess, K.R. Clinical trial design for microarray predictive marker discovery and assessment. Ann. Oncol. 15, 1731–1737 (2004).
King, H.C. & Sinha, A.A. Gene expression profile analysis by DNA microarrays: promise and pitfalls. J. Am. Med. Assoc. 286, 2280–2288 (2001).
Miller, L.D. et al. Optimal gene expression analysis by microarrays. Cancer Cell 2, 353–361 (2002).
Eber, T., Chapman, J. & Shoenfeld, Y. Anti-ribosomal P-protein and its role in psychiatric manifestations of systemic lupus erythematosus: myth or reality? Lupus 14, 571–575 (2005).
Baslund, B. & Petersen, J. Anti-neutrophil cytoplasm autoantibodies (ANCA). The need for specific and sensitive assays. Autoimmunity 27, 231–238 (1998).
Eckel-Passow, J.E., Hoering, A., Therneau, T.M. & Ghobrial, I. Experimental design and analysis of antibody microarrays: applying methods from cDNA arrays. Cancer Res. 65, 2985–2989 (2005).
Yang, I.V. et al. Within the fold: assessing differential expression measures and reproducibility in microarray assays. Genome Biol. 3, research0062 (2002)(doi:10.1186/gb-2002-3-11-research0062).
Wedege, E., Bolstad, K., Wetzler, L.M. & Guttormsen, H. IgG antibody levels to meningococcal porins in patient sera: comparison of immunoblotting and ELISA measurements. J. Immunol. Methods 244, 9–15 (2000).
Satoh, M. et al. Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J. Immunol. 157, 3200–3206 (1996).
Satoh, M. et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin. Exp. Immunol. 121, 399–405 (2000).
Mizutani, A. et al. Pristane-induced autoimmunity in germ-free mice. Clin. Immunol. 114, 110–118 (2005).
Elkon, K. et al. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 83, 7419–7423 (1986).
Mahler, M. et al. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. J. Mol. Med. 81, 194–204 (2003).
Bruner, B.F., Wynn, D.M., Reichlin, M., Harley, J.B. & James, J.A. Humoral antigenic targets of the ribosomal p0 lupus autoantigen are not limited to the carboxyl region. Ann. NY Acad. Sci. 1051, 390–403 (2005).
Tan, P.K. et al. Evaluation of gene expression measurements from commercial microarray platforms. Nucleic Acids Res. 31, 5676–5684 (2003).
Irizarry, R.A. et al. Multiple-laboratory comparison of microarray platforms. Nat. Methods 2, 345–350 (2005).
Bammler, T. et al. Standardizing global gene expression analysis between laboratories and across platforms. Nat Methods 2, 351–356 (2005).
Larkin, J.E., Frank, B.C., Gavras, H., Sultana, R. & Quackenbush, J. Independence and reproducibility across microarray platforms. Nat. Methods 2, 337–344 (2005).
Acknowledgements
We thank S. Chan, J. Tenenbaum and R. Tibshirani and other members of our laboratory for helpful discussion and technical assistance. M.G.K. is funded by the Stanford Medical Scientist Training Program (MSTP). D.L.T. is funded by fellowships from the National Science Foundation and the PEO Sisterhood. I.B. is funded by the Arthritis Foundation Postdoctoral Fellowship. P.J.U. is the recipient of a Donald E. and Delia B. Baxter Foundation Career Development Award and was supported by the Dana Foundation, the Floren Family Trust, the Northern California Chapter of the Arthritis Foundation, National Institutes of Health grants DK61934, AI50854, AI50865 and AR49328, and National Heart, Lung, and Blood Institute (NHLBI) Proteomics contract N01-HV-28183.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
In the past 3 years P.J.U. has served as a consultant to Centocor (Horsham, Pennsylvania, USA), Biogen Idec (Cambridge, Massachusetts, USA), Genentech, Inc. (South San Francisco, California, USA), MedImmune (Gaithersburg, Maryland, USA) and Avanir, Inc. (La Jolla, California, USA), is a member of the Scientific Advisory Board of Monogram Biosciences (South San Francisco, California, USA) and XDx, Inc. (South San Francisco, California, USA), and is a cofounder and consultant at Bayhill Therapeutics (Palo Alto, California, USA). The authors are filing a patent on some of the information described in the article.
Supplementary information
Supplementary Fig. 1
Fold-changes from murine and human dye-swap experiments. (PDF 73 kb)
Supplementary Fig. 2
Cross-labeling of Fab fragments. (PDF 69 kb)
Supplementary Fig. 3
Correlation of single-color and two-color Fab methods with conventional ELISA. (PDF 66 kb)
Supplementary Fig. 4
Anti-ribosomal P reactivity by western-blot and immunoprecipitation in pristane-treated BALB/c mice. (PDF 186 kb)
Supplementary Table 1
Bias of Alexa and Cyanine dyes. (PDF 44 kb)
Supplementary Table 2
Measurement of artificial up- and down-regulation of antibody levels. (PDF 45 kb)
Supplementary Table 3
List of autoantigens and vendors. (PDF 55 kb)
Rights and permissions
About this article
Cite this article
Kattah, M., Alemi, G., Thibault, D. et al. A new two-color Fab labeling method for autoantigen protein microarrays. Nat Methods 3, 745–751 (2006). https://doi.org/10.1038/nmeth910
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth910
This article is cited by
-
Emerging technologies in autoantibody testing for rheumatic diseases
Arthritis Research & Therapy (2017)
-
Systematic reference sample generation for multiplexed serological assays
Scientific Reports (2013)
-
Biosensor analyses of serum autoantibodies: application to antiphospholipid syndrome and systemic lupus erythematosus
Analytical and Bioanalytical Chemistry (2009)
-
HIT: a versatile proteomics platform for multianalyte phenotyping of cytokines, intracellular proteins and surface molecules
Nature Medicine (2008)
-
Technology Insight: can autoantibody profiling improve clinical practice?
Nature Clinical Practice Rheumatology (2007)