Abstract
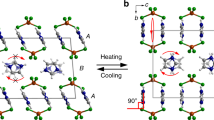
In general, the relatively modest expansion experienced by most materials on heating is caused by increasing anharmonic vibrational amplitudes of the constituent atoms, ions or molecules1. This phenomenon is called positive thermal expansion (PTE) and usually occurs along all three crystallographic axes. In very rare cases, structural peculiarities may give rise either to anomalously large PTE, or to negative thermal expansion (NTE, when lattice dimensions shrink with heating)2,3,4. As NTE and unusually large PTE are extremely uncommon for molecular solids, mechanisms that might give rise to such phenomena are poorly understood. Here we show that the packing arrangement of a simple dumbbell-shaped organic molecule, coupled with its intermolecular interactions, facilitates a cooperative mechanical response of the three-dimensional framework to changes in temperature. A series of detailed structural determinations at 15-K intervals has allowed us to visualize the process at the molecular level. The underlying mechanism is reminiscent of a three-dimensional (3D) folding trellis and results in exceptionally large and reversible uniaxial PTE and biaxial NTE of the crystal. Understanding such mechanisms is highly desirable for the future design of sensitive thermomechanical actuators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ashcroft, N. W. & Mermin, N. D. Solid State Physics (Holt, Rinehart & Winston, 1976).
Goodwin, A. L. et al. Colossal positive and negative thermal expansion in the framework material Ag3[Co(CN)6]. Science 319, 794–797 (2008).
Barrera, G. D., Bruno, J. A. O., Barron, T. H. K. & Allan, N. L. Negative thermal expansion. J. Phys. Condens. Matter 17, R217–R252 (2005).
Jasmine, L. K., Michael, J. K. & Daniel, B. L. Impact of metallophilicity on ‘colossal’ positive and negative thermal expansion in a series of isostructural dicyanometallate coordination polymers. J. Am. Chem. Soc. 131, 4866–4871 (2009).
Khuong, T.-A. V., Nuez, J. E., Godinez, C. E. & Garcia-Garibay, M. A. Crystalline molecular machines: A quest toward solid-state dynamics and function. Acc. Chem. Res. 39, 413–422 (2006).
Evans, J. S. O. Negative thermal expansion materials. J. Chem. Soc. Dalton. Trans. 3317–3326 (1999).
Lightfoot, P., Woodcock, D. A., Maple, M. J., Villaescusa, L. A. & Wright, P. A. The widespread occurrence of negative thermal expansion in zeolites. J. Mater. Chem. 11, 212–216 (2001).
Goodwin, A. L. & Kepert, C. J. Negative thermal expansion and low-frequency modes in cyanide-bridged framework materials. Phys. Rev. B 71, 140301–140304 (2005).
Goodwin, A. L., Chapman, K. W. & Kepert, C. J. Guest-dependent negative thermal expansion in nanoporous Prussian blue analogues MIIPtIV(CN)6·x{H2O} (0≤x≤2; M=Zn, Cd). J. Am. Chem. Soc. 127, 17980–17981 (2005).
Wu, Y. et al. Negative thermal expansion in the metal–organic framework material Cu3(1,3,5−benzenetricarboxylate)2 . Angew. Chem. Int. Ed. 47, 8929–8932 (2008).
Zhou, W., Wu, H., Yildirim, T., Simpson, J. R. & Walker, A. R. H. Origin of the exceptional negative thermal expansion in metal–organic framework-5 Zn4O(1,4−benzenedicarboxylate)3 . Phys. Rev. B 78, 054114 (2008).
White, G. K. & Choy, C. L. Thermal expansion and Grüneisen parameters of isotropic and oriented polyethylene. J. Polym. Sci. Polym. Phys. Ed. 22, 835–846 (1984).
Birkedal, H., Schwarzenbach, D. & Pattison, P. Observation of uniaxial negative thermal expansion in an organic crystal. Angew. Chem. Int. Ed. 41, 754–756 (2002).
Haas, S. et al. Large uniaxial negative thermal expansion in pentacene due to steric hindrance. Phys. Rev. B 76, 205203 (2007).
Krishnan, R. S., Srinivasan, R. & Devanarayanan, S. Thermal Expansion of Crystals (Pergamon, 1979).
Lloyd, G. O. et al. Solid-state self-inclusion: The missing link. Angew. Chem. Int. Ed. 45, 5354–5358 (2006).
Yang, C., Wang, X. & Omary, M. A. Crystallographic observation of dynamic gas adsorption sites and thermal expansion in a breathable fluorous metal–organic framework. Angew. Chem. Int. Ed. 48, 2500–2505 (2009).
McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 3814–3816 (2007).
Nelson, J. B. & Riley, D. P. The thermal expansion of graphite from 15 ∘C to 800 ∘C: Part I. Experimental. Proc. Phys. Soc. Lond. 57, 477–486 (1945).
Arvanitidis, J., Papagelis, K., Margadonna, S., Prassides, K. & Fitch, A. N. Temperature-induced valence transition and associated lattice collapse in samarium fulleride. Nature 425, 599–602 (2003).
Salvador, J. R., Guo, F., Hogan, T. & Kanatzidis, M. G. Zero thermal expansion in YbGaGe due to an electronic valence transition. Nature 425, 702–705 (2003).
Xing, X., Deng, J., Chen, J. & Liu, G. Novel thermal expansion of lead titanate. Rare Metals 22, 294–297 (2003).
Schilfgaarde, M. v., Abrikosov, I. A. & Johansson, B. Origin of the invar effect in iron-nickel alloys. Nature 400, 46–49 (1999).
Tasumi, M. & Simanouchi, T. Crystal vibrations and intermolecular forces of polymethylene crystals. J. Chem. Phys. 43, 1245–1258 (1965).
Maddox, J. Crystal from first principles. Nature 335, 201 (1988).
Dunitz, J. D. Are crystal structures predictable? Chem. Commun. 545–548 (2003).
Acknowledgements
We are grateful to A. Pietraszko of the Institute of Low Temperature and Structure Research of the Polish Academy of Sciences in Wrocław for the X-ray powder diffraction studies. We thank the National Research Foundation and Department of Science and Technology (SARCHI Program) for support of this work. Crystallographic data for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre under reference numbers CCDC 723,906–723,913. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK).
Author information
Authors and Affiliations
Contributions
T.J. synthesized the compound and carried out the initial crystal-structure determinations and thermal analyses. D.D. carried out the variable-temperature studies and wrote the initial draft of the letter. L.J.B. wrote the final draft, prepared the figures and coordinated the project.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 2308 kb)
Supplementary Information
Supplementary Movie 1 (MOV 65 kb)
Supplementary Information
Supplementary Movie 2 (MOV 26 kb)
Supplementary Information
Supplementary Movie 3 (MOV 311 kb)
Supplementary Information
Supplementary Movie 4 (MOV 452 kb)
Supplementary Information
Supplementary Movie 5 (MOV 1214 kb)
Rights and permissions
About this article
Cite this article
Das, D., Jacobs, T. & Barbour, L. Exceptionally large positive and negative anisotropic thermal expansion of an organic crystalline material. Nature Mater 9, 36–39 (2010). https://doi.org/10.1038/nmat2583
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2583
This article is cited by
-
Giant uniaxial negative thermal expansion in FeZr2 alloy over a wide temperature range
Nature Communications (2023)
-
Helical model of compression and thermal expansion
Scientific Reports (2023)
-
Dynamic magnetic crossover at the origin of the hidden-order in van der Waals antiferromagnet CrSBr
Nature Communications (2022)
-
Giant anisotropic thermal expansion actuated by thermodynamically assisted reorientation of imidazoliums in a single crystal
Nature Communications (2019)
-
Rotator side chains trigger cooperative transition for shape and function memory effect in organic semiconductors
Nature Communications (2018)