Abstract
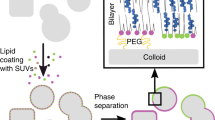
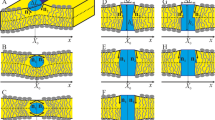
Liquid-ordered (LO) domains reconstituted in model membranes1,2,3,4,5,6 have provided a useful platform for in vitro studies of the lipid-raft model7,8,9, in which signalling membrane molecules are thought to be compartmentalized in sphingolipid- and cholesterol-rich domains. These in vitro studies, however, have relied on an uncontrolled phase-separation process that gives a random distribution of LO domains. Obviously, a precise control of the size and spatial distribution of the LO domains would enable a more systematic large-scale in vitro study of the lipid-raft model. The prerequisite for such capability would be the generation of a well-defined energy landscape for reconstituting the LO domain without disrupting the two-dimensional (2D) fluidity of the model membrane. Here we report controlling the reconstitution of the LO domains in a spatially selective manner by predefining a landscape of energy barriers using topographic surface modifications. We show that the selective reconstitution spontaneously arises from the 2D brownian motion of nanoscale LO domains and signalling molecules captured in these nanodomains, which in turn produce a prescribed, concentrated downstream biochemical process. Our approach opens up the possibility of engineering model biological membranes by taking advantage of the intrinsic 2D fluidity. Moreover, our results indicate that the topographic configuration of cellular membranes could be an important machinery for controlling the lipid raft in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dietrich, C. et al. Lipid rafts reconstituted in model membranes. Biophys. J. 80, 1417–1428 (2001).
Dietrich, C., Volovyk, Z. N., Levi, M., Thompson, N. L. & Jacobson, K. Partitioning of Thy-1, GM1, and cross-linked phospholipids analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc. Natl Acad. Sci. USA 98, 10642–10647 (2001).
Veatch, S. L. & Keller, S. L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89, 268101 (2002).
Baumgart, T., Hess, S. T. & Webb, W. W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 425, 821–824 (2003).
Roux, A. et al. Role of curvature and phase separation in lipid sorting and fission of membrane tubules. EMBO J. 24, 1537–1545 (2005).
Bacia, K., Schwille, P. & Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl Acad. Sci. USA 102, 3272–3277 (2005).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–41 (2000).
Brown, D. A. & London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221–17224 (2000).
Anderson, R. G. W. & Jacobson, K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825 (2002).
Sackmann, E. Supported membranes: scientific and practical applications. Science 271, 43–48 (1996).
Kaizuka, Y. & Groves, J. T. Structure and dynamics of supported intermembrane junctions. Biophys. J. 86, 905–912 (2004).
McConnell, H. M. & Radhakrishnan, A. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta 1610, 159–173 (2003).
Safran, S. A. Statistical Thermodynamics of Surfaces, Interfaces, and Membranes (Addison-Wesley, New York, USA, 1994).
Seifert, U. & Lipowsky, R. Adhesion of vesicles. Phys. Rev. A 42, 4768–4771 (1990).
Helfrich, W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. C 28, 693–703 (1973).
Chen, Z. & Rand, R. P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73, 267–276 (1997).
Keller, C. A., Glasmästar, K., Zhdanov, V. P. & Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett. 84, 5443–5446 (2000).
Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. & Webb, W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Merritt, E. A. et al. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 3, 166–175 (1994).
Lin, J. & Shaw, A. S. Getting downstream without a raft. Cell 121, 815–816 (2006).
McMahon, H. T. & Gallop, J. L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596 (2005).
Braun, D. & Fromherz, P. Fluorescence interferometry of neuronal cell adhesion on microstructured silicon. Phys. Rev. Lett. 81, 5241–5244 (1998).
Buboltz, J. T. & Feigenson, G. W. A novel strategy for the preparation of liposomes: rapid solvent exchange. Biochim. Biophys. Acta 1417, 232–245 (1999).
Acknowledgements
T.-Y.Y. thanks T. Ha and C.-J. Yu for valuable help and discussions, and M. K. Nahas, M. C. McKinney and B. Okumus for critical reading. This work was supported in part by the Korea Science and Engineering Foundation through the Center for Field Responsive Molecules at Korea University and Samsung SDI. M.W.K. acknowledges partial support from DMR-0080034, IMT-2000-B3-2, KISTEP I-03-064 and Korea Health 21 R&D Project of Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary figures S1, S2 and S3 (PDF 139 kb)
Rights and permissions
About this article
Cite this article
Yoon, TY., Jeong, C., Lee, SW. et al. Topographic control of lipid-raft reconstitution in model membranes. Nature Mater 5, 281–285 (2006). https://doi.org/10.1038/nmat1618
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1618
This article is cited by
-
Surface Sensitive Analysis Device using Model Membrane and Challenges for Biosensor-chip
BioChip Journal (2020)
-
Continuity of Monolayer-Bilayer Junctions for Localization of Lipid Raft Microdomains in Model Membranes
Scientific Reports (2016)
-
Reconstituting ring-rafts in bud-mimicking topography of model membranes
Nature Communications (2014)
-
Lipid-nanostructure hybrids and their applications in nanobiotechnology
NPG Asia Materials (2013)
-
Glycans pattern the phase behaviour of lipid membranes
Nature Materials (2013)