Abstract
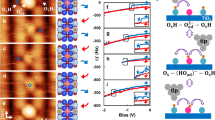
The chemistry of metal oxide surfaces has long been thought to be dominated by reactions involving defects1,2. These are minority sites such as oxygen vacancies. Thus far, it has proved difficult to obtain direct experimental evidence to support this idea, although some progress has been made3,4,5. Here, we use the scanning tunnelling microscope (STM) to image the reaction of water molecules with bridging-oxygen vacancies on a model oxide surface, rutile TiO2(110). In a form of single-molecule chemistry, individual oxygen vacancies are observed being transformed into OH species as a water molecule dissociates in the vacancy. We use the STM tip to selectively desorb individual H atoms, whilst leaving the vacancies intact. This allows us to distinguish between vacancies and OH, which have a similar appearance in STM. In a very clear way, these results validate the view that defects can play a key role in oxide surface reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Henrich, V. E. & Cox, P. A. The Surface Science of Metal Oxides (Cambridge Univ. Press, Cambridge, 1994).
Freund, H.-J. Oxide surfaces. Faraday Discuss. 114, 1–31 (1999).
Henderson, M. A. et al. Interaction of molecular oxygen with the vacuum-annealed TiO2(110) surface: Molecular and dissociative channels. J. Phys. Chem. B 103, 5328–5337 (1999).
Epling, W. S., Peden, C. H. F., Henderson, M. A. & Diebold, U. Evidence for oxygen adatoms on TiO2(110) resulting from O2 dissociation at vacancy sites. Surf. Sci. 412–413, 333–343 (1998).
Aizawa, M. et al. Oxygen vacancy promoting catalytic dehydration of formic acid on TiO2(110) by in situ scanning tunneling microscopic observation. J. Phys. Chem. B 109, 18831–18838 (2005).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Blossey, R. Self-cleaning surfaces — virtual realities. Nature Mater. 2, 301–306 (2003).
Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003).
Onishi, H., Fukui, K. & Iwasawa, Y. Atomic-scale surface structures of TiO2 (110) determined by scanning tunneling microscopy: A new surface-limited phase of titanium oxide. Bull. Chem. Soc. Jpn 68, 2447–2458 (1995).
Diebold, U., Anderson, J. F., Ng, K.-O. & Vanderbilt, D. Evidence for the tunneling site on transition-metal oxides: TiO2(110) . Phys. Rev. Lett. 77, 1322–1325 (1996).
Diebold, U. et al. Intrinsic defects on a TiO2(110) (1×1) surface and their reaction with oxygen: A scanning tunneling microscopy study. Surf. Sci. 411, 137–153 (1998).
Suzuki, S., Fukui, K., Onishi, H. & Iwasawa, Y. Hydrogen adatoms on TiO2(110)-(1×1) characterized by scanning tunneling microscopy and electron stimulated desorption. Phys. Rev. Lett. 84, 2156–2159 (2000).
Schaub, R. et al. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110) . Phys. Rev. Lett. 87, 266104 (2001).
Henderson, M. A. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 46, 1–308 (2002).
Brookes, I. M., Muryn, C. A. & Thornton, G. Imaging water dissociation on TiO2(110) . Phys. Rev. Lett. 87, 266103 (2001).
Lindan, P. J. D. & Zhang, C. Exothermic water dissociation on the rutile TiO2(110) surface. Phys. Rev. B 72, 075439 (2005).
Mayne, A. J., Rose, F. & Dujardin, G. Inelastic interactions of tunnel electrons with surfaces. Faraday Discuss. 117, 241–248 (2000).
Ruscic, B. et al. On the enthalpy of formation of hydroxyl radical and gas-phase bond dissociation energies of water and hydroxyl. J. Phys. Chem. A 106, 2727–2747 (2002).
Shen, T.-C. et al. Atomic-scale desorption through electronic and vibrational-excitation mechanisms. Science 268, 1590–1592 (1995).
Avouris, Ph. et al. Breaking individual chemical bonds via STM-induced excitations. Surf. Sci. 363, 368–377 (1996).
Onda, K. et al. Wet electrons at the H2O /TiO2(110) surface. Science 308, 1154–1158 (2005).
Diebold, U. et al. High transient mobility of chlorine on TiO2(110) : Evidence for “cannon-ball” trajectories of hot adsorbates. Phys. Rev. Lett. 81, 405–408 (1998).
Hofer, W. A., Foster, A. S. & Shluger, A. L. Theories of scanning probe microscopes at the atomic scale. Rev. Mod. Phys. 75, 1287–1331 (2003).
Knotek, M. L. & Feibelman, P. J. Ion desorption by core-hole Auger decay. Phys. Rev. Lett. 40, 964–967 (1978).
Farfan-Arribas, E. & Madix, R. J. Characterization of the acid-base properties of the TiO2(110) surface by adsorption of amines. J. Phys. Chem. B 107, 3225–3233 (2003).
Schaub, R. et al. Oxygen-mediated diffusion of oxygen vacancies on the TiO2(110) surface. Science 299, 377–379 (2003).
Thompson, T. L., Diwald, O. & Yates, J. T. Jr. Molecular oxygen-mediated vacancy diffusion on TiO2(110)-new studies of the proposed mechanism. Chem. Phys. Lett. 393, 28–30 (2004).
Rasmussen, M. D., Molina, L. M. & Hammer, B. Adsorption, diffusion, and dissociation of molecular oxygen at defected TiO2(110) : A density functional theory study. J. Chem. Phys. 120, 988–997 (2004).
Wu, X., Selloni, A., Lazzeri, M. & Nayak, S. K. Oxygen vacancy mediated adsorption and reactions of molecular oxygen on the TiO2(110) surface. Phys. Rev. B 68, 241402 (2003).
Acknowledgements
This work was funded by EPSRC (UK), an EU 5th framework grant (OXIDESURFACES), CREST of JST (Japan) and a Royal Society (UK)–JSPS (Japan) grant for collaboration. C.L.P. is grateful to JSPS (Japan) for the award of a Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bikondoa, O., Pang, C., Ithnin, R. et al. Direct visualization of defect-mediated dissociation of water on TiO2(110) . Nature Mater 5, 189–192 (2006). https://doi.org/10.1038/nmat1592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1592
This article is cited by
-
Mechanistic insight on water dissociation on pristine low-index TiO2 surfaces from machine learning molecular dynamics simulations
Nature Communications (2023)
-
Flower-like MoS2/cotton fiber-derived TiO2 composites with strong electromagnetic wave absorption performance
Journal of Materials Science (2023)
-
In Situ TEM under Optical Excitation for Catalysis Research
Topics in Current Chemistry (2022)
-
Single hydrogen atom manipulation for reversible deprotonation of water on a rutile TiO2 (110) surface
Communications Chemistry (2021)
-
Scanning probe microscopy
Nature Reviews Methods Primers (2021)