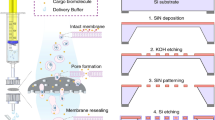
Abstract
Membranes with various pore size, length, morphology and density have been synthesized from diverse materials1,2,3,4,5 for size-exclusion-based separation. An example is the sterilization of intravenous lines by exclusion of bacteria and viruses using polyvinylidene fluoride membranes with 0.1-μm-diameter pores. Chemically specific filtration has recently been addressed for small molecules6,7,8,9,10. Nevertheless, specific bio-organism immobilization and detection remains a great technical challenge in many biomedical applications, such as decontamination or analysis of air and liquids such as drinking water and body fluids. To achieve this goal, materials with controlled pore diameter, length and surface chemistry are required. In this letter, we present the first functionalized silicon membranes and demonstrate their ability to selectively capture simulated bio-organisms. These extremely versatile and rigid devices open the door to a new class of materials that are able to recognize the external fingerprints of bio-organisms—such as size and outer membrane proteins—for specific capture and detection applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Idris, A., Ismail, A.F., Noordin, M.Y. & Shilton, S.J. Optimization of cellulose acetate hollow fiber reverse osmosis membrane production using Taguchi method. J. Membrane Sci. 205, 223– 237 ( 2002).
Martin, C.R. Nanomaterials: a membrane-based synthetic approach. Science 266, 1961– 1966 ( 1994).
Jirage, K.B., Hulteen, J.C. & Martin, C.R. Nanotube-based molecular-filtration membranes. Science 278, 655– 658 ( 1997).
Desai, T.A., Hansford, D. & Ferrari, M. Characterization of micromachined silicon membranes for immunoisolation and bioseparation applications. J. Membrane Sci. 159, 221– 231 ( 1999).
Leoni, L., Boiarski, A. & Desai, T.A. Characterization of nanoporous membranes for immunoisolation: diffusion properties and tissue effects. Biomed. Microdevices 4, 131– 139 ( 2002).
Jirage, K.B., Hulteen, J.C. & Martin, C.R. Effects of thiol chemisorption on the transport properties of gold nanotubule membranes. Anal. Chem. 71, 4913– 4918 ( 1999).
Bok Lee, S. & Martin, C.R. Controlling the transport properties of gold nanotubule membranes using chemisorbed thiols. Chem. Mater. 13, 3236– 3244 ( 2001).
Lee, S.B. et al. Antibody-based bio-nanotube membranes for enantiomeric drug separation. Science 296, 2198– 2200 ( 2002).
Fernandez-Lopez, S. et al. Antibacterial agents based on the cyclic D,L-alpha-peptide architecture. Nature 412, 452– 455 ( 2001).
Chun, K.-Y. & Stroeve, P. Protein transport in nanoporous membranes modified with self assembled monolayers of functionalized thiols. Langmuir 18, 4653– 4658 ( 2002).
Lehmann, V. & Foll, H. Formation mechanism and properties of electrochemically etched trenches in n-type silicon. J. Electrochem. Soc. 137, 653– 659 ( 1990).
Birner, A., Wehrspohn, R.B., Gosele, U.M. & Busch, K. Silicon-based photonic crystals. Adv. Mater. 13, 377– 388 ( 2001).
Lehmann, V., Stengl, R., Reisinger, H., Detemple, R. & Theiss, W. Optical shortpass filters based on macroporous silicon. Appl. Phys. Lett. 78, 589– 591 ( 2001).
Loncar, M., Doll, T., Vuckovic, J. & Scherer, A. Design and fabrication of silicon photonic crystal optical waveguides. J. Lightwave Technol. 18, 1402– 1411 ( 2000).
Lehmann, V. Barcoded molecules. Nature Mater. 1, 12– 13 ( 2002).
Buriak, J.M. Organometallic chemistry on silicon and germanium surfaces. Chem. Rev. 102, 1271– 1308 ( 2002).
Dancil, K.-P.S., Greiner, D.P. & Sailor, M.J. A porous silicon optical biosensor: Detection of reversible binding of IgG to a protein A-modified surface. J. Am. Chem. Soc. 121, 7925– 7930 ( 1999).
Buriak, J.M. & Allen, M.J. Lewis acid mediated functionalization of porous silicon with substituted alkenes and alkynes. J. Am. Chem. Soc. 120, 1339– 1340 ( 1998).
Stewart, M.P. & Buriak, J.M. Exciton-mediated hydrosilylation on photoluminescent nanocrystalline silicon. J. Am. Chem. Soc. 123, 7821– 7830 ( 2001).
Boukherroub, R. et al. Ideal passivation of luminescent porous silicon by thermal, noncatalytic reaction with alkenes and aldehydes. Chem. Mater. 13, 2002– 2011 ( 2001).
Canham, L.T. et al. Derivatized mesoporous silicon with dramatically improved stability in simulated human blood plasma. Adv. Mater. 11, 1505– 1507 ( 1999).
Hart, B.R. et al. New method for attachment of biomolecules to porous silicon. Chem. Comm. 3, 322– 323 ( 2003).
Acknowledgements
This work was performed under the auspices of the US Department of Energy by University of California Lawrence Livermore National Laboratory under contract W-7405-Eng-48. It was funded by a Laboratory Directed Research and Development grant (LDRD-ER # 00-ERD-009). The authors are very grateful to John G. Reynolds for the considerable amount of advice that he provided for the design of the chemical linker.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Membrane functionalization method
Figure 1 Physical and optical properties of dyed polystyrene micro-beads used in this study. (PDF 150 kb)
Figure 2 Permeation of silicon membranes by dyed polystyrene microbeads.
Rights and permissions
About this article
Cite this article
Létant, S., Hart, B., van Buuren, A. et al. Functionalized silicon membranes for selective bio-organism capture. Nature Mater 2, 391–395 (2003). https://doi.org/10.1038/nmat888
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat888
This article is cited by
-
Glucose oxidase immobilized macro porous silicon based conductive glucose sensor
Applied Physics A (2022)
-
Slow-Growing Titanium Dioxide on Ti-48Al Porous Alloy Mediated by Nb and Cr Addition: Perspective via Local Metal–Oxygen Bonding Strength
Journal of Materials Engineering and Performance (2020)