Abstract
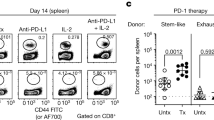
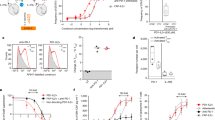
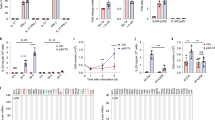
Interleukin (IL)-2 is currently used to enhance T-cell immunity but can have both positive and negative effects on T cells. To determine whether these opposing results are due to IL-2 acting differently on T cells depending on their stage of differentiation, we examined the effects of IL-2 therapy during the expansion, contraction and memory phases of the T-cell response in lymphocytic choriomeningitis virus (LCMV)–infected mice. IL-2 treatment during the expansion phase was detrimental to the survival of rapidly dividing effector T cells. In contrast, IL-2 therapy was highly beneficial during the death phase, resulting in increased proliferation and survival of virus-specific T cells. IL-2 treatment also increased proliferation of resting memory T cells in mice that controlled the infection. Virus-specific T cells in chronically infected mice also responded to IL-2 resulting in decreased viral burden. Thus, timing of IL-2 administration and differentiation status of the T cell are critical parameters in designing IL-2 therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Smith, K.A. Interleukin-2: inception, impact, and implications. Science 240, 1169–1176 (1988).
Gillis, S., Gillis, A.E. & Smith, K.A. The detection of a spleen focus-forming virus neoantigen by lymphocyte-mediated cytolysis. J. Exp. Med. 148, 18–31 (1978).
Van Parijs, L. et al. Uncoupling IL-2 signals that regulate T-cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11, 281–288 (1999).
Gillis, S. & Smith, K.A. Long term culture of tumour-specific cytotoxic T cells. Nature 268, 154–156 (1977).
Akbar, A.N. et al. Interleukin-2 receptor common γ-chain signaling cytokines regulate activated T-cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26, 294–299 (1996).
Lenardo, M.J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 353, 858–861 (1991).
Cousens, L.P., Orange, J.S. & Biron, C.A. Endogenous IL-2 contributes to T-cell expansion and IFN-γ production during lymphocytic choriomeningitis virus infection. J. Immunol. 155, 5690–5699 (1995).
Barouch, D.H. et al. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97, 4192–4197 (2000).
Kuroda, K. et al. Implantation of IL-2-containing osmotic pump prolongs the survival of superantigen-reactive T cells expanded in mice injected with bacterial superantigen. J. Immunol. 157, 1422–1431 (1996).
Sadlack, B. et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993).
Suzuki, H. et al. Deregulated T-cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Willerford, D.M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Dai, Z., Konieczny, B.T. & Lakkis, F.G. The dual role of IL-2 in the generation and maintenance of CD8+ memory T cells. J. Immunol. 165, 3031–3036 (2000).
Ku, C.C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Rosenberg, S.A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Kovacs, J.A. et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 335, 1350–1356 (1996).
Kovacs, J.A. et al. Interleukin-2 induced immune effects in human immunodeficiency virus-infected patients receiving intermittent interleukin-2 immunotherapy. Eur. J. Immunol. 31, 1351–1360 (2001).
Davey, R.T. et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA 284, 183–189 (2000).
Jacobson, E.L., Pilaro, F. & Smith, K.A. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc. Natl. Acad. Sci. USA 93, 10405–10410 (1996).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Matloubian, M., Kolhekar, S.R., Somasundaram, T. & Ahmed, R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 67, 7340–7349 (1993).
Zajac, A.J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Kaech, S.M. & Ahmed, R. Memory CD8+ T-cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
Wong, P. & Pamer, E.G. Antigen independent CD8 T-cell proliferation. J. Immunol. 166, 5864–5868 (2001).
van Stipdonk, M.J., Lemmens, E.E. & Schoenberger, S.P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2, 381–382 (2001).
von Herrath, M.G., Yokoyama, M., Dockter, J., Oldstone, M.B. & Whitton, J.L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70, 1072–1079 (1996).
Lau, L.L., Jamieson, B.D., Somasundaram, T. & Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 369, 648–652 (1994).
Grayson, J.M., Murali-Krishna, K., Altman, J.D. & Ahmed, R. Gene expression in antigen-specific CD8+ T cells during viral infection. J. Immunol. 166, 795–799 (2001).
Murali-Krishna, K. & Ahmed, R. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165, 1733–1737 (2000).
Lotze, M.T. et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 135, 2865–2875 (1985).
Lotze, M.T. et al. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer 58, 2764–2772 (1986).
Mier, J.W. et al. Induction of circulating tumor necrosis factor (TNF α) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J. Clin. Immunol. 8, 426–436 (1988).
Callan, M.F. et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187, 1395–1402 (1998).
Wilson, J.D. et al. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS 14, 225–233 (2000).
Jin, X. et al. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J. Infect. Dis. 181, 165–175 (2000).
Komanduri, K.V. et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1- infected subjects. Virology 279, 459–470 (2001).
Zhang, X., Sun, S., Hwang, I., Tough, D.F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Smith, K. Interleukin 2 immunotherapy. in Therapeutic Immunology (eds. Austen, F., Burakoff, S., Rosen, F. & Strom, T.) 240–250 (Blackwell Science, Oxford, UK, 2001).
Smith, K.A. et al. In vivo assessment of antiviral reactivity in chronic HIV infection. HIV Clin. Trials 1, 16–22 (2000).
Smith, K.A. To cure chronic HIV infection, a new therapeutic strategy is needed. Curr. Opin. Immunol. 13, 617–624 (2001).
Yee, C., Riddell, S.R. & Greenberg, P.D. Prospects for adoptive T-cell therapy. Curr. Opin. Immunol. 9, 702–708 (1997).
Whitmire, J.K. et al. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T-cell responses but is dispensable for CD8 T-cell responses. J. Immunol. 163, 3194–3201 (1999).
Acknowledgements
We thank P.L. Mahar and other members of the Ahmed lab for helpful suggestions and technical assistance, and B.D. Evavold, P.D. Greenberg and L.E. Cheng for critical reading of this manuscript. This work was supported by National Institutes of Health grants AI30048 (to R.A.) and A151181 (to K.A.S.), the Cancer Research Institute/James E. Siegel Perpetual Fellowship Award (to J.N.B.), National Research Service Award 1F32AI0249-01A1 (to J.M.G.), a fellowship from the Cancer Research Institute (to E.J.W.), and the Cancer Research Fund of the Damon Runyon Cancer Research Foundation (DRG-1570 to S.M.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Blattman, J., Grayson, J., Wherry, E. et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 9, 540–547 (2003). https://doi.org/10.1038/nm866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm866
This article is cited by
-
Longitudinal association of cytokine-producing CMV-specific T cells with frailty in HIV-infected and -uninfected men who have sex with men
Immunity & Ageing (2022)
-
Immunobiology and pathogenesis of hepatitis B virus infection
Nature Reviews Immunology (2022)
-
Restoration of HBV-specific CD8+ T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-α therapy
Signal Transduction and Targeted Therapy (2021)
-
Lentiviral vector induces high-quality memory T cells via dendritic cells transduction
Communications Biology (2021)
-
Interleukin-2 and regulatory T cells in rheumatic diseases
Nature Reviews Rheumatology (2021)