Abstract
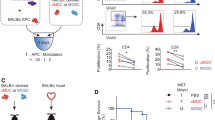
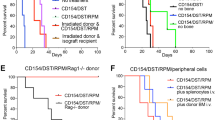
Successful transplantation of allogeneic organs is an important objective in modern medicine. However, sophisticated immune defense mechanisms, primarily evolved to combat infections, often work against medical transplantation. To investigate the roles of natural and adaptive immune responses in transplant rejection, we functionally inactivated key effector systems of the innate (NK cells) and the adaptive immune system (CD28-mediated costimulation of T cells) in mice. Neither of these interventions alone led to acceptance of allogeneic vascularized cardiac grafts. In contrast, inhibition of NK-receptor–bearing cells combined with CD28-costimulation blockade established long-term graft acceptance. These results indicate a concerted interplay between innate and adaptive immune surveillance for graft rejection. Thus we suggest that inactivation of NK-receptor–bearing cells could be a new strategy for successful survival of solid-organ transplants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Medzhitov, R. & Janeway, C.A. Jr. An ancient system of host defense. Curr. Opin. Immunol. 10, 12–15 (1998).
Karre, K. & Colonna, M. Specificity, Function, and Development of NK cells. (Springer, London, 1998).
Correa, I. & Raulet, D.H. Binding of diverse peptides to MHC class I molecules inhibits target cell lysis by activated natural killer cells. Immunity 2, 61–71 (1995).
Lanier, L.L. The role of natural killer cells in transplantation. Curr. Opin. Immunol. 7, 626–631 (1995).
Ljunggren, H.G. & Karre, K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990).
Ortaldo, J.R., Winkler-Pickett, R., Mason, A.T. & Mason, L.H. The Ly-49 family: regulation of cytotoxicity and cytokine production in murine CD3+ cells. J. Immunol. 160, 1158–1165 (1998).
Skold, M. & Cardell, S. Differential regulation of Ly49 expression on CD4+ and CD4-CD8- (double negative) NK1.1+ T cells. Eur. J. Immunol. 30, 2488–2496 (2000).
Biron, C.A. & Welsh, R.M. Blastogenesis of natural killer cells during viral infection in vivo. J. Immunol. 129, 2788–2795 (1982).
del Val, M. et al. Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial-Golgi compartment. J. Exp. Med. 176, 729–738 (1992).
Jones, T.R. et al. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69, 4830–4841 (1995).
Colonna, M. Specificity and function of immunoglobulin superfamily NK cell inhibitory and stimulatory receptors. Immunol. Rev. 155, 127–133 (1997).
Yu, Y.Y., Kumar, V. & Bennett, M. Murine natural killer cells and marrow graft rejection. Annu. Rev. Immunol. 10, 189–213 (1992).
Heidecke, C.D. et al. Lack of evidence for an active role for natural killer cells in acute rejection of organ allografts. Transplantation 40, 441–444 (1985).
Zinkernagel, R.M. & Doherty, P.C. The discovery of MHC restriction. Immunol. Today 18, 14–17 (1997).
Gould, D.S. & Auchincloss, H., Jr. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol. Today 20, 77–82 (1999).
Warrens, A.N., Lombardi, G. & Lechler, R.I. Presentation and recognition of major and minor histocompatibility antigens. Transpl. Immunol. 2, 103–107 (1994).
Lafferty, K.J., Prowse, S.J., Simeonovic, C.J. & Warren, H.S. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu. Rev. Immunol. 1, 143–173 (1983).
Abbas, A.K. & Janeway, C.A. Jr. Immunology: improving on nature in the twenty-first century. Cell 100, 129–138 (2000).
Schwartz, R.H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 71, 1065–1068 (1992).
Boussiotis, V.A., Freeman, G.J., Gribben, J.G. & Nadler, L.M. The role of B7-1/B7-2:CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol. Rev. 153, 5–26 (1996).
Johnson, J.G. & Jenkins, M.K. Accessory cell-derived signals required for T cell activation. Immunol. Res. 12, 48–64 (1993).
Shahinian, A. et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261, 609–612 (1993).
Kawai, K., Shahinian, A., Mak, T.W. & Ohashi, P.S. Skin allograft rejection in CD28-deficient mice. Transplantation 61, 352–355 (1996).
Szot, G.L. et al. Absence of host B7 expression is sufficient for long-term murine vascularized heart allograft survival. Transplantation 69, 904–909 (2000).
Lin, H. et al. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J. Exp. Med. 188, 199–204 (1998).
Klein, J. Immunology. The Science of Self-Nonself Discrimination. 476–479 (John Wiley & Sons, New York, 1982).
Pearson, T.C. et al. Transplantation tolerance induced by CTLA4-Ig. Transplantation 57, 1701–1706 (1994).
Razi-Wolf, Z. et al. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc. Natl. Acad. Sci. USA 89, 4210–4214 (1992).
Turka, L.A. et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA 89, 11102–11105 (1992).
Hale, D.A., Gottschalk, R., Maki, T. & Monaco, A.P. Use of CTLA4-Ig in combination with conventional immunosuppressive agents to prolong allograft survival. Transplantation 64, 897–900 (1997).
Larsen, C.P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Li, Y. et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Med. 5, 1298–1302 (1999).
Trambley, J. et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest 104, 1715–1722 (1999).
Dharnidharka, V.R., Schowengerdt, K. & Skoda-Smith, S. Failure of combined costimulatory blockade in animal transplant model. Nature Med. 6, 115 (2000).
Honey, K., Cobbold, S.P. & Waldmann, H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J. Immunol. 163, 4805–4810 (1999).
Newell, K.A. et al. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J. Immunol. 163, 2358–2362 (1999).
Kimachi, K., Croft, M. & Grey, H.M. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur. J. Immunol. 27, 3310–3317 (1997).
Reay, P.A. et al. Determination of the relationship between T cell responsiveness and the number of MHC-peptide complexes using specific monoclonal antibodies. J. Immunol. 164, 5626–5634 (2000).
Manning, T.C. et al. Antigen recognition and allogeneic tumor rejection in CD8+ TCR transgenic/RAG(−/−) mice. J. Immunol. 159, 4665–4675 (1997).
Shelton, M.W., Walp, L.A., Basler, J.T., Uchiyama, K. & Hanto, D.W. Mediation of skin allograft rejection in scid mice by CD4+ and CD8+ T cells. Transplantation 54, 278–286 (1992).
Shi, F.D. et al. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nature Immunol. 1, 245–251 (2000).
Li, Y., Zheng, X.X., Li, X.C., Zand, M.S. & Strom, T.B. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation 66, 1387–1388 (1998).
Karre, K. & Welsh, R.M. Viral decoy vetoes killer cell. Nature 386, 446–447 (1997).
Corry, R.J., Winn, H.J. & Russell, P.S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16, 343–350 (1973).
Endres, R. et al. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 7, 419–432 (1997).
Acknowledgements
We thank K. Mink and U. Huffstatt for technical assistance; Y. Chvatcho and M. Kosco-Vilbois for the antibody against NK1.1; R. Endres, N. Zantl, T. Plitz, and H. Neubauer for helpful discussions; N. Zantl for help with microsurgical techniques; and H. Wagner and J.-R. Siewert for continuous and generous support. Grants for these studies was provided by the DFG (259/2-4 to K.P. and Si208/ 5-1/ SFB576 to K.P. and C.-D.H.). This work forms part of an M.D. thesis of C. Tertilt and N. Chambron.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maier, S., Tertilt, C., Chambron, N. et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med 7, 557–562 (2001). https://doi.org/10.1038/87880
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/87880
This article is cited by
-
Sterile inflammation in thoracic transplantation
Cellular and Molecular Life Sciences (2021)
-
Immunological challenges associated with artificial skin grafts: available solutions and stem cells in future design of synthetic skin
Journal of Biological Engineering (2017)
-
Macrophages as Effectors of Acute and Chronic Allograft Injury
Current Transplantation Reports (2016)
-
Microbiota—implications for immunity and transplantation
Nature Reviews Nephrology (2015)
-
Peripheral natural killer cell and allo-stimulated T-cell function in kidney transplant recipients associate with cancer risk and immunosuppression-related complications
Kidney International (2015)