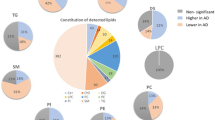
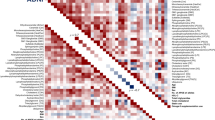
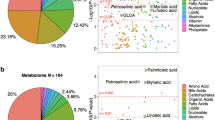
Abstract
Alzheimer's disease causes a progressive dementia that currently affects over 35 million individuals worldwide and is expected to affect 115 million by 2050 (ref. 1). There are no cures or disease-modifying therapies, and this may be due to our inability to detect the disease before it has progressed to produce evident memory loss and functional decline. Biomarkers of preclinical disease will be critical to the development of disease-modifying or even preventative therapies2. Unfortunately, current biomarkers for early disease, including cerebrospinal fluid tau and amyloid-β levels3, structural and functional magnetic resonance imaging4 and the recent use of brain amyloid imaging5 or inflammaging6, are limited because they are either invasive, time-consuming or expensive. Blood-based biomarkers may be a more attractive option, but none can currently detect preclinical Alzheimer's disease with the required sensitivity and specificity7. Herein, we describe our lipidomic approach to detecting preclinical Alzheimer's disease in a group of cognitively normal older adults. We discovered and validated a set of ten lipids from peripheral blood that predicted phenoconversion to either amnestic mild cognitive impairment or Alzheimer's disease within a 2–3 year timeframe with over 90% accuracy. This biomarker panel, reflecting cell membrane integrity, may be sensitive to early neurodegeneration of preclinical Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
Change history
20 June 2014
In the version of this article initially published online, the Source Data file for Figure 1 contained a transposition error that occurred when the authors were moving data from their analysis software into Excel. This error does not affect the accuracy of the data shown in Figure 1. This error has been corrected in the HTML version of the article.
References
World Health Organization. Dementia: a Public Health Priority (World Health Organization, Geneva, 2012).
Sperling, R.A. et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: the Journal of the Alzheimer's Association 7, 280–292 (2011).
Hulstaert, F. et al. Improved discrimination of AD patients using β-amyloid(1–42) and tau levels in CSF. Neurology 52, 1555–1562 (1999).
Small, S.A., Perera, G.M., De La Paz, R., Mayeux, R. & Stern, Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann. Neurol. 45, 466–472 (1999).
Klunk, W.E. et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 55, 306–319 (2004).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Thambisetty, M. & Lovestone, S. Blood-based biomarkers of Alzheimer's disease: challenging but feasible. Biomark. Med. 4, 65–79 (2010).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 58, 267–288 (1996).
Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning; Data Mining, Inference, and Prediction. (Springer-Verlag, New York, 2008).
van Meer, G. & de Kroon, A.I. Lipid map of the mammalian cell. J. Cell Sci. 124, 5–8 (2011).
Jones, L.L., McDonald, D.A. & Borum, P.R. Acylcarnitines: role in brain. Prog. Lipid Res. 49, 61–75 (2010).
Nitsch, R.M. et al. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 89, 1671–1675 (1992).
Schaefer, E.J. et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 63, 1545–1550 (2006).
Mulder, C. et al. Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer's disease. J. Neural Transm. 110, 949–955 (2003).
Walter, A. et al. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol. Aging 25, 1299–1303 (2004).
Prasad, M.R., Lovell, M.A., Yatin, M., Dhillon, H. & Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem. Res. 23, 81–88 (1998).
Pettegrew, J.W., Panchalingam, K., Hamilton, R.L. & McClure, R.J. Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem. Res. 26, 771–782 (2001).
Haughey, N.J., Bandaru, V.V., Bae, M. & Mattson, M.P. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim. Biophys. Acta 1801, 878–886 (2010).
Kordower, J.H. & Fiandaca, M.S. Response of the monkey cholinergic septohippocampal system to fornix transection: a histochemical and cytochemical analysis. J. Comp. Neurol. 298, 443–457 (1990).
Kordower, J.H. et al. The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc. Natl. Acad. Sci. USA 91, 10898–10902 (1994).
Whitehouse, P.J., Price, D.L., Clark, A.W., Coyle, J.T. & DeLong, M.R. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126 (1981).
Hansson, O. et al. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 5, 228–234 (2006).
Blennow, K., Hampel, H., Weiner, M. & Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144 (2010).
Irizarry, M.C. Biomarkers of Alzheimer disease in plasma. NeuroRx 1, 226–234 (2004).
Ray, S. et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat. Med. 13, 1359–1362 (2007).
Doecke, J.D. et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch. Neurol. 69, 1318–1325 (2012).
Roe, C.M. et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer disease. Neurology 76, 501–510 (2011).
Fagan, A.M. et al. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 64, 343–349 (2007).
Albert, M.S. et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279 (2011).
Petersen, R.C. et al. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308 (1999).
Espinosa, A. et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J. Alzheimers Dis. 34, 769–780 (2013).
McKhann, G.M. et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 263–269 (2011).
Want, E.J. et al. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 5, 1005–1018 (2010).
Grebe, S.K. & Singh, R.J. LC-MS/MS in the clinical laboratory - where to from here? Clin. Biochem. Rev. 32, 5–31 (2011).
Illig, T. et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 42, 137–141 (2010).
Römisch-Margl, W.P.C., Bogumil, R., Röhring, C. & Suhre, K. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics 7, 1–14 (2011).
Bolstad, B.M., Irizarry, R.A., Astrand, M. & Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003).
Ma, S. & Huang, J. Regularized ROC method for disease classification and biomarker selection with microarray data. Bioinformatics 21, 4356–4362 (2005).
Liu, Z. & Tan, M. ROC-based utility function maximization for feature selection and classification with applications to high-dimensional protease data. Biometrics 64, 1155–1161 (2008).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Acknowledgements
We thank E. Johnson and D. Greenia for study coordination; P. Bailie, M. Patel and A. Balasubramanian for assistance with data collection; and A. Almudevar for statistical support. We also thank R. Padilla and I. Conteh for processing the blood samples and R. Singh and P. Kaur for technical assistance in developing the lipidomics data. This work was funded by US National Institutes of Health grants R01AG030753 and DOD W81XWH-09-1-0107 to H.J.F.
Author information
Authors and Affiliations
Contributions
H.J.F., T.R.M., C.H.K., W.J.H., S.G.F., M.M., M.S.F. and A.K.C. conceived of the study. W.J.H., J.M.H., M.D.N., S.A.R. and C.H.K. recruited participants and provided material support for data collection. M.M., D.J.B. and C.B.P. collected the clinical data. D.R.P., S.G.F. and M.M. derived the cognitive z-score methodology. M.M. completed statistical analysis of the cognitive data. A.K.C., M.S.F., T.R.M. and L.H.M. completed the lipidomics analyses. M.T.T., X.Z. and A.K.C. completed statistical analysis of the lipidomics data. M.M., A.K.C., M.S.F. and H.J.F. wrote the manuscript. All authors edited the manuscript for content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3, Supplementary Figures 1–4 and Supplementary Note. (PDF 1035 kb)
Source data
Rights and permissions
About this article
Cite this article
Mapstone, M., Cheema, A., Fiandaca, M. et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 20, 415–418 (2014). https://doi.org/10.1038/nm.3466
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3466
This article is cited by
-
Plasma metabolic profiles predict future dementia and dementia subtypes: a prospective analysis of 274,160 participants
Alzheimer's Research & Therapy (2024)
-
Brain areas lipidomics in female transgenic mouse model of Alzheimer's disease
Scientific Reports (2024)
-
Lipidomics profiling reveals distinct patterns of plasma sphingolipid alterations in Alzheimer’s disease and vascular dementia
Alzheimer's Research & Therapy (2023)
-
Roles of bile acids signaling in neuromodulation under physiological and pathological conditions
Cell & Bioscience (2023)
-
Cerebrospinal fluid camk2a levels at baseline predict long-term progression in multiple sclerosis
Clinical Proteomics (2023)