Abstract
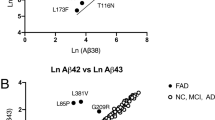
To determine whether the presenilin 1 (PS1), presenilin 2 (PS2) and amyloid β-protein precursor (APP) mutations linked to familial Alzheimer's disease (FAD) increase the extracellular concentration of amyloid β–protein (Aβ) ending at Aβ42(43) in vivo, we performed a blinded comparison of plasma Aβ levels in carriers of these mutations and controls. Aβ1 –42(43) was elevated in plasma from subjects with FAD–linked PS1 (P < 0.0001), PS2N141I (P = 0.009), APPK670N,M671L (P < 0.0001), and APPV717I (one subject) mutations. Aβ ending at Aβ42(43) was also significantly elevated in fibroblast media from subjects with PS1 (P < 0.0001) or P52 (P = 0.03) mutations. These findings indicate that the FAD–linked mutations may all cause Alzheimer's disease by increasing the extracellular concentration of Aβ42(43), thereby fostering cerebral deposition of this highly amyloidogenic peptide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Iwatsubo, T., Mann, D.M., Odaka, A., Suzuki, N. & Ihara, Y. Amyloid β protein (Aβ) deposition: Aβp42(43) precedes Aβ40 in Down syndrome. Ann. Neurol. 37, 294–299 (1995).
Gravina, S.A. et al. Amyloid beta protein (Aβ) in Alzheimer's disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ40 or Aβ42(43). J. Biol. Chem. 270, 7013–7016 (1995).
Roher, A. et al. Structural alterations in the peptide backbone of β-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J. Biol. Chem. 268, 3072–3083 (1993).
Miller, D.L. et al. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch. Biochem. 301, 41–52 (1993).
Seubert, P. et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature 359, 325–327 (1992).
Shoji, M. et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258, 126–129 (1992).
Haass, C. et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Busciglio, J., Gabuzda, D.H., Matsudaira, P. & Yankner, B.A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc. Natl. Acad. Sci. USA 90, 2092–2096 (1993).
Dovey, H.F., Suomesaari-Chrysler, S., Lieberburg, I., Sinha, S. & Kiem, P.S. Cells with a familial Alzheimer's disease mutation produce authentic β-peptide. Neuro Report 4, 1039–1042(1993).
Vigo-Pelfrey, C., Lee, D., Keim, P., Lieberburg, I. & Schenk, D.B. Characterization of p amyloid peptide from human cerebrospinal fluid. J. Neurochem. 61, 19965–19968 (1993).
Hilbich, C., Kisters-Woike, B., Reed, J., Masters, C.L. & Beyreuther, K. Aggregation and secondary structure of synthetic amyloid βA4 peptides of Alzheimer's disease. J. Mol. Biol. 218, 149–163 (1991).
Burdick, D. et al. Assembly and aggregation properties of synthetic Alzheimer's A4/β amyloid peptide analogs. J. Biol. Chem. 267, 546–554 (1992).
Jarrett, J.T. & Lansbury, P.T., Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73, 1055–1058 (1993).
Jarrett, J.T., Berger, E.P. & Lansbury, P.T., The carboxy terminus of p amyloid protein is critical for the seeding of amyloid formation: Implications for patho-genesis of Alzheimer's disease. Biochemistry 32, 4693–4697 (1993).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
Levy-Lahad, E. et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269, 973–977 (1995).
Levy-Lahad, E. et al. A familial Alzheimer's disease locus on chromosome 1. Science 269, 970–973 (1995).
Rogaev, E. et al. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature 376, 775–778 (1995).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349, 704–706 (1991).
Naruse, S. et al. Missense mutation Val-Ile in exon 17 of amyloid precursor protein gene in Japanese familial Alzheimer's disease. Lancet 337, 978 (1991).
Yoshioka, K., Miki, T., Katsuya, T., Ogihara, T. & Sakaki, Y. The 717Val-Ile substitution in amyloid precursor protein is associated with familial Alzheimer's disease regardless of ethnic groups. Biochem. Biophys. Res. Commun. 178, 1141 (1991).
Hardy, J. et al. Molecular classification of Alzheimer's disease. Lancet 337, 1342 (1991).
Murrell, J., Farlow, M., Ghetti, B. & Benson, M.D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science 254, 97–99 (1991).
Chartier-Harlin, M.-C. et al. Early-onset Alzheimer's disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 353, 844–846 (1991).
Mullan, M. et al. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of β-amyloid. Nature Genet. 1, 345–347 (1992).
Citron, M. et al. Excessive production of amyloid beta-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc. Natl. Acad. Sci. USA 91, 11993–11997 (1994).
Cai, X.D., Golde, T.E. & Younkin, S.G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 259, 514–516 (1993).
Citron, M. et al. Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases p-protein production. Nature 360, 672–674 (1992).
Suzuki, N. et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (P APP717) mutants. Science 264, 1336–1340 (1994).
Tamaoka, A. et al. APP717 missense mutation affects the ratio of amyloid beta protein species (Aβl-42/43 and Aβ1-40) in familial Alzheimer's disease brain. J. Biol. Chem. 269, 32721–32724 (1994).
Motter, R. et al. Reduction of beta-amyloid peptide 42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann. Neurol. 38, 643–648 (1995).
Grubb, A. et al. Abnormal metabolism of gamma-trace alkaline microprotein. The basic defect in hereditary cerebral hemorrhage with amyloidosis. N. Engl. J. Med. 311, 1547–1549 (1984).
Ma, J., Yee, A., Brewer, H.B., Jr., Das, S. & Potter, H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature 372, 92–94 (1994).
Wisniewski, T., Castano, E.M., Golabek, A., Vogel, T. & Frangione, B. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am. J. Pathol. 145, 1030–1035 (1994).
Castaño, E. et al. Fibrillogenesis in Alzheimer's disease of the amyloid beta peptides and apolipoprotein E. Biochem. J. 306, 599–604 (1995).
Evans, K.C., Berger, E.P., Cho, C.G., Weisgraber, K.H. & Lansbury, P.T., Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatment of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 92, 763–767 (1995).
Larson, E.B. et al. University of Washington Alzheimer's Disease Patient Registry (ADPR): 1987–1988. Aging 2, 404–408 (1990).
Kukull, W.A. et al. Solvent exposure as a risk factor for Alzheimer's disease: A case-control study. Am. J. Epidemiol. 141, 1059–1071 (1995); (erratum) Am. J. Epidemiol. 142, 450 (1996).
McKhann, G. et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCSDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34, 939–944 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scheuner, D., Eckman, C., Jensen, M. et al. Secreted amyloid β–protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med 2, 864–870 (1996). https://doi.org/10.1038/nm0896-864
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0896-864
This article is cited by
-
Sleep fragmentation affects glymphatic system through the different expression of AQP4 in wild type and 5xFAD mouse models
Acta Neuropathologica Communications (2023)
-
Simple model systems reveal conserved mechanisms of Alzheimer’s disease and related tauopathies
Molecular Neurodegeneration (2023)
-
Five-mer peptides prevent short-term spatial memory deficits in Aβ25-35-induced Alzheimer’s model mouse by suppressing Aβ25-35 aggregation and resolving its aggregate form
Alzheimer's Research & Therapy (2023)
-
Emerging diagnostics and therapeutics for Alzheimer disease
Nature Medicine (2023)
-
Scallop-derived plasmalogen attenuates amyloid beta-induced inflammation and apoptosis in SH-SY5Y cells
Molecular & Cellular Toxicology (2023)