Abstract
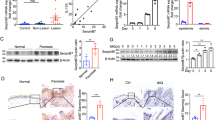
Acne rosacea is an inflammatory skin disease that affects 3% of the US population over 30 years of age and is characterized by erythema, papulopustules and telangiectasia1,2,3. The etiology of this disorder is unknown, although symptoms are exacerbated by factors that trigger innate immune responses, such as the release of cathelicidin antimicrobial peptides4. Here we show that individuals with rosacea express abnormally high levels of cathelicidin in their facial skin and that the proteolytically processed forms of cathelicidin peptides found in rosacea are different from those present in normal individuals. These cathelicidin peptides are a result of a post-translational processing abnormality associated with an increase in stratum corneum tryptic enzyme (SCTE) in the epidermis. In mice, injection of the cathelicidin peptides found in rosacea, addition of SCTE, and increasing protease activity by targeted deletion of the serine protease inhibitor gene Spink5 each increases inflammation in mouse skin. The role of cathelicidin in enabling SCTE-mediated inflammation is verified in mice with a targeted deletion of Camp, the gene encoding cathelicidin. These findings confirm the role of cathelicidin in skin inflammatory responses and suggest an explanation for the pathogenesis of rosacea by demonstrating that an exacerbated innate immune response can reproduce elements of this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kelly, A.P. Acne and related disorders. in Principles and Practice of Dermatology (eds. Sams, W. & Lynch, P.) 801–818 (Churchill Livingstone, New York, 1996).
Straus, J. Rosacea and acne rosacea. in Dermatology (eds. Orkin, M., Maibach, H. & Dahl, M.) 337–338 (Prentice Hall International Inc., New Jersey, 1991).
Crawford, G.H., Pelle, M.T. & James, W.D. Rosacea: I. Etiology, pathogenesis, and subtype classification. J. Am. Acad. Dermatol. 51, 327–341 quiz 342–344 (2004).
Zanetti, M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 7, 179–196 (2005).
Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75, 39–48 (2004).
van Dijk, A., Veldhuizen, E.J., van Asten, A.J. & Haagsman, H.P. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Vet. Immunol. Immunopathol. 106, 321–327 (2005).
Chang, C.I., Pleguezuelos, O., Zhang, Y.A., Zou, J. & Secombes, C.J. Identification of a novel cathelicidin gene in the rainbow trout, Oncorhynchus mykiss. Infect. Immun. 73, 5053–5064 (2005).
Nizet, V. et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 (2001).
Rosenberger, C.M., Gallo, R.L. & Finlay, B.B. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 101, 2422–2427 (2004).
Iimura, M. et al. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 174, 4901–4907 (2005).
Howell, M.D. et al. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 172, 1763–1767 (2004).
Ong, P.Y. et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347, 1151–1160 (2002).
Oppenheim, J.J. & Yang, D. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 17, 359–365 (2005).
De, Y. et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074 (2000)[AG1].
Koczulla, R. et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Invest. 111, 1665–1672 (2003).
Gallo, R.L. et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA 91, 11035–11039 (1994).
Frohm, M. et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272, 15258–15263 (1997).
Heilborn, J.D. et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 120, 379–389 (2003).
Zaiou, M., Nizet, V. & Gallo, R.L. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Invest. Dermatol. 120, 810–816 (2003).
Braff, M.H. et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J. Immunol. 174, 4271–4278 (2005).
Yamasaki, K. et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicdiins in skin. FASEB J. 20, 2068–2080 (2006).
Murakami, M., Lopez-Garcia, B., Braff, M., Dorschner, R.A. & Gallo, R.L. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 172, 3070–3077 (2004).
Descargues, P. et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat. Genet. 37, 56–65 (2005).
Gallo, R.L. et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272, 13088–13093 (1997).
Komatsu, N. et al. Quantification of human tissue kallikreins in the stratum corneum: dependence on age and gender. J. Invest. Dermatol. 125, 1182–1189 (2005).
Dorschner, R.A. et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 117, 91–97 (2001).
Acharya, M.R., Venitz, J., Figg, W.D. & Sparreboom, A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist. Updat. 7, 195–208 (2004).
Hansson, L. et al. Epidermal overexpression of stratum corneum chymotryptic enzyme in mice: a model for chronic itchy dermatitis. J. Invest. Dermatol. 118, 444–449 (2002).
Brinnel, H., Friedel, J., Caputa, M., Cabanac, M. & Grosshans, E. Rosacea: disturbed defense against brain overheating. Arch. Dermatol. Res. 281, 66–72 (1989).
Murakami, M. et al. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Invest. Dermatol. 119, 1090–1095 (2002).
Acknowledgements
We thank B. Cottrell for instructions in SELDI-TOF-MS analysis. This work was supported by the National Institutes of Health (R01-AI052453, R01-AR45676), The National Rosacea Society and a VA Merit Award (R.L.G.); and the Association for Preventive Medicine of Japan (K.Y.).
Author information
Authors and Affiliations
Contributions
K.Y. conducted SELDI-TOF-MS experiments, protease assays including in situ zymography and in vivo studies, and wrote the manuscript. A.D.N. conducted the in vivo skin irritation model. A.B., M.M. and T.O. performed immunohistochemistry, dot blot and in situ hybridization. A.C. performed immunofluorescence. R.A.D. prepared and purified peptides. C.B., P.D. and A.H. contributed to the experiments with Spink5-deficient mice. A.B., V.B.M. and R.L.G. organized human sample collection. R.L.G. conceived, designed and supervised all aspects of this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4, Supplementary Methods (PDF 886 kb)
Rights and permissions
About this article
Cite this article
Yamasaki, K., Di Nardo, A., Bardan, A. et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 13, 975–980 (2007). https://doi.org/10.1038/nm1616
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1616
This article is cited by
-
A Review of the Diagnostic and Therapeutic Gaps in Rosacea Management: Consensus Opinion
Dermatology and Therapy (2024)
-
Colchicine Alleviates Rosacea by Inhibiting Neutrophil Inflammation Activated by the TLR2 Pathway
Inflammation (2024)
-
Whole genome sequencing identifies genetic variants associated with neurogenic inflammation in rosacea
Nature Communications (2023)
-
Mast cell stabilization: new mechanism underlying the therapeutic effect of intense pulsed light on rosacea
Inflammation Research (2023)
-
Individuelle Therapie und Hautpflege
Deutsche Dermatologie (2022)