Abstract
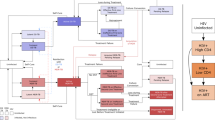
'Hot zones' are areas that have >5% prevalence (or incidence) of multidrug-resistant tuberculosis (MDRTB). We present a new mathematical model (the amplifier model) that tracks the emergence and evolution of multiple (pre-MDR, MDR and post-MDR) strains of drug-resistant Mycobacterium tuberculosis. We reconstruct possible evolutionary trajectories that generated hot zones over the past three decades, and identify the key causal factors. Results are consistent with recently reported World Health Organization (WHO) data. Our analyses yield three important insights. First, paradoxically we found that areas with programs that successfully reduced wild-type pansensitive strains often evolved into hot zones. Second, some hot zones emerged even when MDR strains were substantially less fit (and thus less transmissible) than wild-type pansensitive strains. Third, levels of MDR are driven by case-finding rates, cure rates and amplification probabilities. To effectively control MDRTB in the hot zones, it is essential that the WHO specify a goal for minimizing the amplification probability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Iseman, M.D. Evolution of drug-resistant tuberculosis: a tale of two species. Proc. Natl. Acad. Sci. USA 91, 2428–2429 (1994).
Iseman, M.D. Tuberculosis therapy: past, present and future. Eur. Respir. J. 36, 87s–94s (2002).
Reichman, L. & Tanne, J.H. Timebomb: The Global Epidemic of Multi-Drug-Resistant Tuberculosis (McGraw-Hill, New York, 2002).
Dye, C., Espinal, M.A., Watt, C.J., Mbiaga, C. & Williams, B.G. Worldwide incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 185, 1197–1202 (2002).
Espinal, M.A. et al. Global trends in resistance to antituberculosis drugs. World Health Organization—International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N. Engl. J. Med. 344, 1294–1303 (2001).
Pablos-Méndez, A. et al. Anti-tuberculosis drug resistance in the world. Report 1 (World Health Organization, Geneva, 2000).
Farmer, P.E., Reichman, L. & Iseman, M.D. Report: The global impact of drug-resistant tuberculosis (Harvard Medical School, Open Society Institute, Cambridge, Massachusetts, USA, 1999).
Espinal, M.A. et al. Anti-tuberculosis drug resistance in the world. Report no. 2: Prevalence and trends (World Health Organization, Geneva, 2003).
Farmer, P.E. et al. The dilemma of MDR-TB in the global era. Int. J. Tuberc. Lung. Dis. 2, 869–876 (1998).
Mitnick, C. et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N. Engl. J. Med. 348, 119–128 (2003).
Blower, S.M. & Daley, C.L. Problems and solutions for the Stop TB partnership. Lancet Infect. Dis. 2, 374–376 (2002).
Espinal, M.A. Time to abandon the standard retreatment regimen with first-line drugs for failures of standard treatment. Int. J. Tuberc. Lung. Dis. 7, 607–608 (2003).
Blower, S.M., Small, P.M. & Hopewell, P.C. Control strategies for tuberculosis epidemics: new models for old problems. Science 273, 497–500 (1996).
Blower, S.M. & Gerberding, J.L. Understanding, predicting and controlling the emergence of drug-resistant tuberculosis: a theoretical framework. J. Mol. Med. 76, 624–636 (1998).
Blower, S.M., Porco, T.C. & Lietman, T. Tuberculosis: the evolution of antibiotic resistance and the design of epidemic control strategies. in Mathematical Models in Medical and Health Sciences (eds. Horn, Simonett & Webb) (Vanderbilt University Press, Nashville, TN, 1998).
Blower, S.M., Koelle, K. & Lietman, T. Antibiotic resistance—to treat... Nat. Med. 5, 358 (1999).
Castillo-Chavez, C. & Feng, Z. To treat or not to treat: the case of tuberculosis. J. Math. Biol. 35, 629–656 (1997).
Dye, C. & Williams, B.G. Criteria for the control of drug-resistant tuberculosis. Proc. Natl. Acad. Sci. USA 97, 8180–8185 (2000).
Hopewell, P.C. Tuberculosis control: how the world has changed since 1990. Bull. World Health Organ. 80, 427 (2002).
Frieden, T., Sterling, T., Munsiff, S., Watt, C.J. & Dye, C. Tuberculosis. Lancet 362, 887–899 (2003).
Blower, S.M. & Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Inter. Stat. Rev. 2, 229–243 (1994).
Blower, S.M. et al. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1, 815–821 (1995).
Sanchez, E. & Blower, S.M. Uncertainty and sensitivity analysis of the basic reproductive rate. Tuberculosis as an example. Am. J. Epidemiol. 145, 1127–1137 (1997).
Porco, T.C. & Blower, S.M. Quantifying the intrinsic transmission dynamics of tuberculosis. Theo. Pop. Bio. 54, 117–132 (1998).
Blower, S.M., Porco, T.C. & Darby, G. Predicting and preventing the emergence of antiviral drug resistance in HSV-2. Nat. Med. 4, 673–678 (1998).
Bankes, S. Tools and techniques for developing policies for complex and uncertain systems. Proc. Natl. Acad. Sci. USA 99 (Suppl. 3), 7263–7266 (2002).
Elzinga, G., Raviglione, M.C. & Maher, D. Scale up: meeting targets in global tuberculosis control. Lancet 363, 814–819 (2004).
Mukherjee, J.S. et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 363, 474–481 (2004).
Lietman, T. & Blower, S.M. The potential impact of tuberculosis vaccines as epidemic control agents. Clin. Infect. Dis. 30, S316–S322 (2000).
Porco, T.C., Small, P.M. & Blower, S.M. Amplification dynamics: Predicting the effect of HIV on tuberculosis outbreaks. J. Acquir. Immune Defic. Syndr. 28, 437–444 (2001).
Ziv, E., Daley, C.L. & Blower, S.M. Early therapy for latent tuberculosis infection. Am. J. Epidemiol. 153, 381–385 (2001).
Sterling, T., Lehmann, H. & Frieden, T. Impact of DOTS compared with DOTS-plus on multidrug resistant tuberculosis and tuberculosis deaths: decision analysis. BMJ 326, 574 (2003).
Centers for Disease Control. Primary multidrug-resistant tuberculosis—Ivanovo Oblast, Russia, 1999. MMWR 48, 661–663 (1999).
Migliori, G. et al. Frequency of recurrence among MDR-TB cases 'successfully' treated with standardised short-course chemotherapy. Int. J. Tuberc. Lung. Dis. 6, 858–864 (2002).
Suarez, P. et al. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet 359, 1980–1989 (2002).
Cohen, T., Sommers, B. & Murray, M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect. Dis. 3, 13–21 (2003).
Acknowledgements
The authors gratefully acknowledge L. Ma for coding and assistance with numerical analyses, R. Smith for technical discussions and E. Bodine for editorial assistance. S.M.B. acknowledges financial support from NIAID/NIH (R01 AI041935). T.C. acknowledges financial support from the National Science Foundation through grant DMS-0206733. S.M.B. is extremely grateful to P. Farmer and to J. Kim for many discussions, over many years, of the transmission dynamics of MDRTB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Blower, S., Chou, T. Modeling the emergence of the 'hot zones': tuberculosis and the amplification dynamics of drug resistance. Nat Med 10, 1111–1116 (2004). https://doi.org/10.1038/nm1102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1102
This article is cited by
-
Modeling and dynamic analysis of tuberculosis in mainland China from 1998 to 2017: the effect of DOTS strategy and further control
Theoretical Biology and Medical Modelling (2020)
-
Characteristics of compensatory mutations in the rpoC gene and their association with compensated transmission of Mycobacterium tuberculosis
Frontiers of Medicine (2020)
-
Have compensatory mutations facilitated the current epidemic of multidrug-resistant tuberculosis?
Emerging Microbes & Infections (2018)
-
Global dynamics of a tuberculosis transmission model with age of infection and incomplete treatment
Advances in Difference Equations (2017)
-
The impact of drug resistance on Mycobacterium tuberculosis physiology: what can we learn from rifampicin?
Emerging Microbes & Infections (2014)