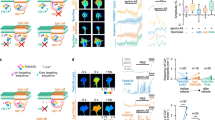
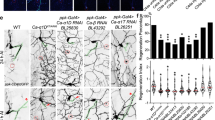
Abstract
Calcium arising through release from intracellular stores and from influx across the plasma membrane is essential for signalling by specific guidance cues and by factors that inhibit axon regeneration. The mediators of calcium influx in these cases are largely unknown. Transient receptor potential channels (TRPCs) belong to a superfamily of Ca2+-permeable, receptor-operated channels that have important roles in sensing and responding to changes in the local environment. Here we report that XTRPC1, a Xenopus homolog of mammalian TRPC1, is required for proper growth cone turning responses of Xenopus spinal neurons to microscopic gradients of netrin-1, brain-derived neurotrophic factor and myelin-associated glycoprotein, but not to semaphorin 3A. Furthermore, XTRPC1 is required for midline guidance of axons of commissural interneurons in the developing Xenopus spinal cord. Thus, members of the TRPC family may serve as a key mediator for the Ca2+ influx that regulates axon guidance during development and inhibits axon regeneration in adulthood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tessier-Lavigne, M. & Goodman, C.S. The molecular biology of axon guidance. Science 274, 1123–1133 (1996).
Yu, T.W. & Bargmann, C.I. Dynamic regulation of axon guidance. Nat. Neurosci. 4 suppl: 1169–1176 (2001).
Huber, A.B., Kolodkin, A.L., Ginty, D.D. & Cloutier, J.F. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26, 509–563 (2003).
Song, H. & Poo, M. The cell biology of neuronal navigation. Nat. Cell Biol. 3, E81–E88 (2001).
Bandtlow, C.E., Schmidt, M.F., Hassinger, T.D., Schwab, M.E. & Kater, S.B. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science 259, 80–83 (1993).
He, Z. & Koprivica, V. The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 27, 341–368 (2004).
Sivasankaran, R. et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 7, 261–268 (2004).
Fournier, A.E. & Strittmatter, S.M. Repulsive factors and axon regeneration in the CNS. Curr. Opin. Neurobiol. 11, 89–94 (2001).
Schwab, M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 14, 118–124 (2004).
Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713 (2003).
Gomez, T.M. & Spitzer, N.C. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J. Neurobiol. 44, 174–183 (2000).
Henley, J. & Poo, M.M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 14, 320–330 (2004).
Hong, K., Nishiyama, M., Henley, J., Tessier-Lavigne, M. & Poo, M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403, 93–98 (2000).
Henley, J.R., Huang, K.H., Wang, D. & Poo, M.M. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44, 909–916 (2004).
Ming, G.L. et al. cAMP-dependent growth cone guidance by netrin-1. Neuron 19, 1225–1235 (1997).
Song, H. et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515–1518 (1998).
Song, H.J., Ming, G.L. & Poo, M.M. cAMP-induced switching in turning direction of nerve growth cones. Nature 388, 275–279 (1997).
Zheng, J.Q. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature 403, 89–93 (2000).
Berridge, M.J. Neuronal calcium signaling. Neuron 21, 13–26 (1998).
Gomez, T.M. & Spitzer, N.C. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 397, 350–355 (1999).
Wong, S.T. et al. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 5, 1302–1308 (2002).
Wen, Z., Guirland, C., Ming, G.L. & Zheng, J.Q.A. CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron 43, 835–846 (2004).
Jin, M. et al. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J. Neurosci. 25, 2338–2347 (2005).
Nishiyama, M. et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423, 990–995 (2003).
Montell, C., Birnbaumer, L. & Flockerzi, V. The TRP channels, a remarkably functional family. Cell 108, 595–598 (2002).
Moran, M.M., Xu, H. & Clapham, D.E. TRP ion channels in the nervous system. Curr. Opin. Neurobiol. 14, 362–369 (2004).
Li, H.S., Xu, X.Z. & Montell, C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24, 261–273 (1999).
Kim, S.J. et al. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426, 285–291 (2003).
Bezzerides, V.J., Ramsey, I.S., Kotecha, S., Greka, A. & Clapham, D.E. Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 6, 709–720 (2004).
Greka, A., Navarro, B., Oancea, E., Duggan, A. & Clapham, D.E. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat. Neurosci. 6, 837–845 (2003).
Bobanovic, L.K. et al. Molecular cloning and immunolocalization of a novel vertebrate trp homologue from Xenopus. Biochem. J. 340, 593–599 (1999).
Brereton, H.M., Harland, M.L., Auld, A.M. & Barritt, G.J. Evidence that the TRP-1 protein is unlikely to account for store-operated Ca2+ inflow in Xenopus laevis oocytes. Mol. Cell. Biochem. 214, 63–74 (2000).
Hedgecock, E.M., Culotti, J.G. & Hall, D.H. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61–85 (1990).
Harris, R., Sabatelli, L.M. & Seeger, M.A. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17, 217–228 (1996).
Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994).
Mitchell, K.J. et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203–215 (1996).
Serafini, T. et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996).
Kennedy, T.E., Serafini, T., de la Torre, J.R. & Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435 (1994).
Li, Y. et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898 (2005).
Gordon, X.W. & Poo, M. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434, 898–904 (2005).
Clapham, D.E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Kolodziej, P.A. et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 (1996).
Keino-Masu, K. et al. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996).
Roberts, A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res. Bull. 53, 585–593 (2000).
Phelps, P.E., Alijani, A. & Tran, T.S. Ventrally located commissural neurons express the GABAergic phenotype in developing rat spinal cord. J. Comp. Neurol. 409, 285–298 (1999).
Roberts, A., Dale, N., Ottersen, O.P. & Storm-Mathisen, J. Development and characterization of commissural interneurones in the spinal cord of Xenopus laevis embryos revealed by antibodies to glycine. Development 103, 447–461 (1988).
Ming, G., Henley, J., Tessier-Lavigne, M., Song, H. & Poo, M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29, 441–452 (2001).
Ming, G. et al. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron 23, 139–148 (1999).
Ren, X.R. et al. Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 7, 1204–1212 (2004).
Sayers, L.G. et al. Intracellular targeting and homotetramer formation of a truncated inositol 1,4,5-trisphosphate receptor-green fluorescent protein chimera in Xenopus laevis oocytes: evidence for the involvement of the transmembrane spanning domain in endoplasmic reticulum targeting and homotetramer complex formation. Biochem. J. 323, 273–280 (1997).
Acknowledgements
We would like to thank A. Kolodkin, C. Montell and T. Dawson for critical comments, N. Marsh-Armstrong, L.N. Borodinsky and N.C. Spitzer for their help during this study, and L. Liu for her technical support. This work was supported by National Institute of Neurological Disorders and Stroke, Charles E. Culpeper Scholarships in Medical Science, Whitehall Foundation, and Basal O'Connor Starter Scholar Research Award Program to G.L.M. S.S. is partially supported by a postdoctoral fellowship from the Korea Science and Engineering Foundation. H.L.R and M.D.B. would like to gratefully acknowledge support from the Biotechnology and Biological Sciences Research Council and Royal Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Neurite extension in the presence or absence of gradients of guidance cues. (a) Summary of neurite extension rates during the turning assay in a gradient of netrin-1 (5 g/ml in the pipette) for different neurons. Values represent mean ± s.e.m. (n = 20-29). (b) Summary of neurite length of neurons derived from embryos injected with either the XTRPC1 morpholino or a control morpholino cultured without the addition of any guidance cues. Values represent mean ± s.e.m. (n = 25-27). No statistical significance was found (P > 0.05, Bootstrap test). (JPG 150 kb)
Supplementary Fig. 2
Density of commissural interneurons in the developing spinal cord of Xenopus embryos. (a-d) Sample projections of Z-stack confocal images of 3A10 staining (red) of Xenopus spinal cord (stage 30) from the sagittal views. The same segment of spinal cord was imaged from the side injected with the XTRPC1 morpholino and fixable FITC-dextran as a lineage tracer (green) as shown in (a) and from the uninjected side as shown in (c). Enlarged images of 3A10 staining were shown in (b, d). The cell bodies of commissural interneurons exhibit specific 3A10 staining, which is not as strong as those for axons. Note a reduction of 3A10+ fiber tracks in the un-injected side when compared to that in the injected side from these sagittal views. Scale bar: 100 m for (a,c) and 40 m for (b,d). (e) Quantification of the density of commissural interneurons in stage 30 embryos. The number of 3A10+ labelled cell bodies was counted from the uninjected and injected side within the same segment of the spinal cord of embryos unilaterally injected with the XTRPC1 morpholino at the two-cell stage. Values represent mean ± s.e.m. (n = 6). No statistical significance was found (P > 0.05, Bootstrap test) (JPG 209 kb)
Supplementary Fig. 3
A model of the involvement of TRPCs in cytoplasmic signalling of guidance cues. (JPG 145 kb)
Supplementary Video 1
3D reconstruction of Z-series confocal images of the longitudinal axonal tracks of commissural interneurons in the ventral portion of the developing Xenopus spinal cord of stage 30 embryos injected with XTRPC1 morpholino. Shown are axons of commissural interneurons stained with monoclonal antibody 3A10 (red). The XTRPC1 morpholino was delivered to half of the embryo together with fixable FITC-dextran as a lineage tracer (green; see Methods). (MOV 2494 kb)
Supplementary Video 2
3D reconstruction of Z-series confocal images of the longitudinal axonal tracks of commissural interneurons in the ventral portion of the developing Xenopus spinal cord of stage 30 embryos injected with control morpholino. Shown are axons of commissural interneurons stained with monoclonal antibody 3A10 (red). Control morpholino was delivered to half of the embryo together with fixable FITC-dextran as a lineage tracer (green; see Methods). (MOV 2494 kb)
Rights and permissions
About this article
Cite this article
Shim, S., Goh, E., Ge, S. et al. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci 8, 730–735 (2005). https://doi.org/10.1038/nn1459
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1459
This article is cited by
-
TRPC5 regulates axonal outgrowth in developing retinal ganglion cells
Laboratory Investigation (2020)
-
TRPC6-Mediated Ca2+ Entry Essential for the Regulation of Nano-ZnO Induced Autophagy in SH-SY5Y Cells
Neurochemical Research (2020)
-
Trpc1 as the Missing Link Between the Bmp and Ca2+ Signalling Pathways During Neural Specification in Amphibians
Scientific Reports (2019)
-
TRPC3 Is Dispensable for β-Alanine Triggered Acute Itch
Scientific Reports (2017)
-
TRPC Channels: Prominent Candidates of Underlying Mechanism in Neuropsychiatric Diseases
Molecular Neurobiology (2016)