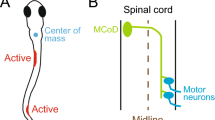
Abstract
Two zebrafish motoneurons, CaP and VaP, are initially developmentally equivalent; later, CaP innervates ventral muscle, whereas VaP dies. Current models suggest that vertebrate motoneuron death results from failure to compete for limited, target-derived trophic support. In contrast, we provide evidence that zebrafish ventral muscle can support both CaP and VaP survival. However, VaP's growth cone is prevented from extending into ventral muscle by CaP-dependent interactions with identified muscle fibers, the muscle pioneers; this interaction breaks the initial equivalence of CaP and VaP. Thus, the processes mediating VaP death are more complex than failure to compete for trophic support, and may be important for correct spatial patterning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Horvitz, H. R., Shaham, S. & Hengartner, M. O. The genetics of programmed cell death in the nematode Caenorhabditis elegans. Cold Spring Harbor Symp. Quant. Biol. 59, 377–385 (1994).
Hamburger, V. & Oppenheim, R. W. Naturally occurring neuronal death in vertebrates. Neurosci. Comm. 1, 39–55 (1982).
Pettmann, B. & Henderson, C. E. Neuronal cell death. Neuron 20, 633–647 (1998).
Grieshammer, U., Lewandoski, M., Prevette, D. M., Oppenheim, R. W. & Martin, G. R. Muscle-specific cell ablation conditional upon Cre-mediated DNA recombination in transgenic mice leads to massive spinal and cranial motoneuron loss. Dev. Biol. 197, 234–247 (1998).
Oppenheim, R. W. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 12, 252–257 (1989).
Greensmith, L. & Vrbová, G. Motoneurone survival: a functional approach. Trends Neurosci. 19, 450–455 (1996).
DeChiara, T. M. et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits. Cell 83, 313–322 (1995).
Garcès, A. et al. GFRα1 is required for development of distinct subpopulations of motoneuron. J. Neurosci. 20, 4992–5000 (2000).
Oppenheim, R. W. et al. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J. Neurosci. 20, 5001–5011 (2000).
Oppenheim, R. W. et al. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J. Neurosci. 21, 1283–1291 (2001).
Westerfield, M., McMurray, J. & Eisen, J. S. Identified motoneurons and their innervation of axial muscles in the zebrafish. J. Neurosci. 6, 2267–2277 (1986).
Eisen, J. S., Pike, S. H. & Romancier, B. An identified neuron with variable fates in embryonic zebrafish. J. Neurosci. 10, 34–43 (1990).
Eisen, J. S. The role of interactions in determining cell fate of two identified motoneurons in the embryonic zebrafish. Neuron 8, 231–240 (1992).
Appel, B. et al. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development 121, 4117–4125 (1995).
Eisen, J. S., Myers, P. Z. & Westerfield, M. Pathway selection by growth cones of identified motoneurons in live zebrafish embryos. Nature 320, 269–271 (1986).
Liu, D. W. & Westerfield, M. The formation of terminal fields in the absence of competitive interactions among primary motoneurons in the zebrafish. J. Neurosci. 10, 3947–3959 (1990).
Melançon, E., Liu, D., Westerfield, M. & Eisen, J. Pathfinding by identified zebrafish motoneurons in the absence of muscle pioneers. J. Neurosci. 17, 7796–7804 (1997).
Beattie, C. E., Melancon, E. & Eisen, J. S. Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons. Development 127, 2653–2662 (2000).
Kimble, J., Sulston, J. & White, J. in Cell Lineage, Stem Cells and Cell Determination (ed. Le Douarin, N.) 59–68 (Elsevier/North Holland Biomedical, Amsterdam, The Netherlands, 1979).
Raible, D. W. & Eisen, J. S. Lateral specification of cell fate during vertebrate development. Curr. Opin. Gen. Dev. 5, 444–449 (1995).
Gatchalian, C. L. & Eisen, J. S. Pathway selection by growth cones of ectopic motoneurons in embryonic zebrafish. Neuron 9, 105–112 (1992).
Stewart, W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14, 741–759 (1978).
Felsenfeld, A. L., Curry, M. & Kimmel, C. B. The fub-1 mutation blocks initial myofibril formation in zebrafish muscle pioneer cells. Dev. Biol. 148, 23–30 (1991).
Halpern, M. E., Ho, R. K., Walker, C. & Kimmel, C. B. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75, 99–111 (1993).
Currie, P. D. & Ingham, P. W. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature 382, 452–455 (1996).
Du, S. J., Devoto, S. H., Westerfield, M. & Moon, R. T. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-β gene families. J. Cell Biol. 139, 145–156 (1997).
Henion, P. D. et al. A screen for mutations affecting development of zebrafish neural crest. Dev. Gen. 18, 11–17 (1996).
Eisen, J. S. Determination of primary motoneuron identity in developing zebrafish embryos. Science 252, 569–572 (1991).
Raible, D. W. et al. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev. Dyn. 195, 29–42 (1992).
Ramirez-Weber, F. A. & Kornberg, T. B. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599–607 (1999).
Pike, S. H. & Eisen, J. S. Interactions between identified motoneurons in embryonic zebrafish are not required for normal motoneuron development. J. Neurosci. 10, 44–49 (1990).
Eisen, J. S. Patterning motoneurons in the vertebrate nervous system. Trends Neurosci. 22, 321–326 (1999).
Wang, H. & Tessier-Lavigne, M. En passant neurotrophic action of an intermediate axonal target in the developing mammalian CNS. Nature 401, 765–769 (1999).
Landmesser, L. in From Message to Mind: Directions in Developmental Neurobiology (eds. Easter, S. S. Jr., Barald, K. F. & Carlson, B. M.) 121–133 (Sinauer Associates, Sunderland, Massachusetts, 1988).
Heinrich, G. & Lum, T. Fish neurotrophins and Trk receptors. Int. J. Dev. Neurosci. 18, 1–27 (2000).
Weisblat, D. A. & Blair, S. S. Developmental indeterminacy in neurogenesis. Phil. Trans. R. Soc. Lond. B Biol. Sci. 312, 39–56 (1984).
Keleher, G. P. & Stent, G. S. Cell position and developmental fate in leech embryogenesis. Proc. Natl. Acad. Sci. USA 87, 8457–8461 (1990).
Huang, F. Z. & Weisblat, D. A. Cell fate determination in an annelid equivalence group. Development 122, 1839–1847 (1996).
Priess, J. R. & Thomson, J. N. Cellular interactions in early C. elegans embryos. Cell 48, 241–250 (1987).
Tanabe, Y. & Jessell, T. M. Diversity and pattern in the developing spinal cord. Science 274, 1115–1123 (1996).
Pfaff, S. & Kintner, C. Neuronal diversification: development of motor neuron subtypes. Curr. Opin. Neurobiol. 8, 27–36 (1998).
Eisen, J. S. Zebrafish make a big splash. Cell 87, 969–977 (1996).
Amsterdam, A. et al. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 13, 2713–2724 (1999).
Beattie, C. E., Raible, D. W., Henion, P. D. & Eisen, J. S. in Methods in Cell Biology: The Zebrafish, Genetics and Genomics Vol. 60 (eds. Detrich, W., Zon, L. & Westerfield, M.) 71–86 (Academic, San Diego, California, 1999).
Granato, M. et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399–413 (1996).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Eisen, J. S., Pike, S. H. & Debu, B. The growth cones of identified motoneurons in embryonic zebrafish select appropriate pathways in the absence of specific cellular interactions. Neuron 2, 1097–1104 (1989).
Eisen, J. S. & Pike, S. H. The spt-1 mutation alters the segmental arrangement and axonal development of identified neurons in the spinal cord of the embryonic zebrafish. Neuron 6, 767–776 (1991).
Acknowledgements
Special thanks to B. Eisen. We thank M. Westerfield, C. Kimmel and D. Armstrong for discussions and comments on the manuscript and the University of Oregon Zebrafish Facility staff for animal husbandry. Supported by NIH NS23915 and HD22486. Renovation and expansion of the University of Oregon Zebrafish Facility supported by NIH RR11724, NSF 9602828, the M.J. Murdock Charitable Trust and the W.M. Keck Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eisen, J., Melançon, E. Interactions with identified muscle cells break motoneuron equivalence in embryonic zebrafish. Nat Neurosci 4, 1065–1070 (2001). https://doi.org/10.1038/nn742
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn742
This article is cited by
-
Zebrafish Mnx proteins specify one motoneuron subtype and suppress acquisition of interneuron characteristics
Neural Development (2012)
-
The zebrafish as a model organism for the study of apoptosis
Apoptosis (2010)
-
Stepping stone to death
Nature Neuroscience (2001)