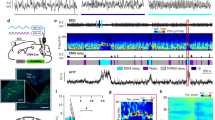
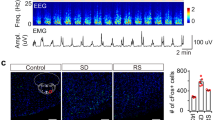
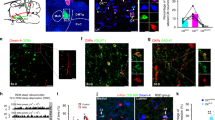
Abstract
Work in animals and humans has suggested the existence of a slow wave sleep (SWS)-promoting/electroencephalogram (EEG)-synchronizing center in the mammalian lower brainstem. Although sleep-active GABAergic neurons in the medullary parafacial zone (PZ) are needed for normal SWS, it remains unclear whether these neurons can initiate and maintain SWS or EEG slow-wave activity (SWA) in behaving mice. We used genetically targeted activation and optogenetically based mapping to examine the downstream circuitry engaged by SWS-promoting PZ neurons, and we found that this circuit uniquely and potently initiated SWS and EEG SWA, regardless of the time of day. PZ neurons monosynaptically innervated and released synaptic GABA onto parabrachial neurons, which in turn projected to and released synaptic glutamate onto cortically projecting neurons of the magnocellular basal forebrain; thus, there is a circuit substrate through which GABAergic PZ neurons can potently trigger SWS and modulate the cortical EEG.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
28 August 2014
In the version of this article initially published, the colors of the data points were reversed relative to the key in Figure 3d, left panel. The error has been corrected in the HTML and PDF versions of the article.
References
Saper, C.B., Fuller, P.M., Pedersen, N.P., Lu, J. & Scammell, T.E. Sleep state switching. Neuron 68, 1023–1042 (2010).
Sherin, J.E., Shiromani, P.J., McCarley, R.W. & Saper, C.B. Activation of ventrolateral preoptic neurons during sleep. Science 271, 216–219 (1996).
Batini, C., Moruzzi, G., Palestini, M., Rossi, G.F. & Zanchetti, A. Persistent patterns of wakefulness in the pretrigeminal midpontine preparation. Science 128, 30–32 (1958).
Affanni, J., Marchiafava, P.L. & Zernicki, B. Higher nervous activity in cats with midpontine pretrigeminal transections. Science 137, 126–127 (1962).
Bonvallet, M. & Bloch, V. Bulbar control of cortical arousal. Science 133, 1133–1134 (1961).
Dang-Vu, T.T. et al. Spontaneous neural activity during human slow wave sleep. Proc. Natl. Acad. Sci. USA 105, 15160–15165 (2008).
Lu, J., Greco, M.A., Shiromani, P. & Saper, C.B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 20, 3830–3842 (2000).
Vyazovskiy, V.V. & Tobler, I. The temporal structure of behaviour and sleep homeostasis. PLoS ONE 7, e50677 (2012).
Borbely, A.A. & Achermann, P. Sleep Homeostsis and Models of Sleep Regulation. in Principles and Practice of Sleep Medicine (eds. Kryger, M.H., Roth, T. & Dement, W.C.) 405–417 (Elsevier Saunders, 2005).
Marshall, L., Helgadottir, H., Molle, M. & Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613 (2006).
Bushey, D., Tononi, G. & Cirelli, C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 332, 1576–1581 (2011).
Massimini, M., Huber, R., Ferrarelli, F., Hill, S. & Tononi, G. The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870 (2004).
Anaclet, C. et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J. Neurosci. 32, 17970–17976 (2012).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Tobler, I. & Borbely, A.A. Sleep EEG in the rat as a function of prior waking. Electroencephalogr. Clin. Neurophysiol. 64, 74–76 (1986).
Tobler, I. Phylogeny of sleep regulation. in Principles and Practice of Sleep Medicine (eds. Kryger, M.H., Roth, T. & Dement, W.C.) 77–90 (Elsevier Saunders, 2005).
Jones, B.E. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog. Brain Res. 145, 157–169 (2004).
Fuller, P.M., Sherman, D., Pedersen, N.P., Saper, C.B. & Lu, J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 519, 933–956 (2011).
Kaur, S. et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J. Neurosci. 33, 7627–7640 (2013).
Saper, C.B. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J. Comp. Neurol. 222, 313–342 (1984).
Franken, P., Tobler, I. & Borbely, A.A. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci. Lett. 130, 141–144 (1991).
Lu, J. et al. Role of endogenous sleep-wake and analgesic systems in anesthesia. J. Comp. Neurol. 508, 648–662 (2008).
Huber, R., Deboer, T. & Tobler, I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 857, 8–19 (2000).
Franken, P., Malafosse, A. & Tafti, M. Genetic determinants of sleep regulation in inbred mice. Sleep 22, 155–169 (1999).
Brown, R.E., Basheer, R., McKenna, J.T., Strecker, R.E. & McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 92, 1087–1187 (2012).
Fuller, P.M., Saper, C.B. & Lu, J. The pontine REM switch: past and present. J. Physiol. (Lond.) 584, 735–741 (2007).
Peyron, C. et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015 (1998).
Sherin, J.E., Elmquist, J.K., Torrealba, F. & Saper, C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 18, 4705–4721 (1998).
Chou, T.C. et al. Afferents to the ventrolateral preoptic nucleus. J. Neurosci. 22, 977–990 (2002).
Vetrivelan, R., Fuller, P.M., Yokota, S., Lu, J. & Saper, C.B. Metabolic effects of chronic sleep restriction in rats. Sleep 35, 1511–1520 (2012).
Magnes, J., Moruzzi, G. & Pompeiano, O. Synchronization of the EEG produced by low-frequency electrical stimulation of the region of the solitary tract. Arch. Ital. Biol. 99, 33–41 (1961).
Reinoso–Barbero, F. & de Andres, I. Effects of opioid microinjections in the nucleus of the solitary tract on the sleep-wakefulness cycle states in cats. Anesthesiology 82, 144–152 (1995).
Laguzzi, R., Reis, D.J. & Talman, W.T. Modulation of cardiovascular and electrocortical activity through serotonergic mechanisms in the nucleus tractus solitarius of the rat. Brain Res. 304, 321–328 (1984).
Puizillout, J.J. Nucleus tractus solitarius, serotonin and regulation of vigilance. Rev. Electroencephalogr. Neurophysiol. Clin. 16, 95–106 (1986).
Nosjean, A., Arluison, M. & Laguzzi, R.F. Increase in paradoxical sleep after destruction of serotoninergic innervation in the nucleus tractus solitarius of the rat. Neuroscience 23, 469–481 (1987).
Schachter, S.C. & Saper, C.B. Vagus nerve stimulation. Epilepsia 39, 677–686 (1998).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Paxinos, G.T. & Franklin, K. The Mouse Brain in Stereotaxic Coordinates 2nd edn. (Academic Press, 2001).
Fuller, P.M., Sherman, D., Pedersen, N.P., Saper, C.B. & Lu, J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 519, 933–956 (2011).
Anaclet, C., Pedersen, N.P., Fuller, P.M. & Lu, J. Brainstem circuitry regulating phasic activation of trigeminal motoneurons during REM sleep. PLoS ONE 5, e8788 (2010).
Anaclet, C. et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J. Neurosci. 32, 17970–17976 (2012).
Alexander, G.M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein–coupled receptors. Neuron 63, 27–39 (2009).
Valatx, J.L., Henane, R., Destombes, J.J. & Perenom, G. Effects of chronic heat exposure on the wakefulness-sleep cycle in the albino rat: 30 degrees C ambient temperature. C R Seances Soc. Biol. Fil. 165, 1660–1664 (1971).
Valatx, J.L. & Bugat, R. Genetic factors as determinants of the waking-sleep cycle in the mouse. Brain Res. 69, 315–330 (1974).
Gooley, J.J., Schomer, A. & Saper, C.B. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 9, 398–407 (2006).
Lu, J., Sherman, D., Devor, M. & Saper, C.B. A putative flip-flop switch for control of REM sleep. Nature 441, 589–594 (2006).
Xiao, X., Li, J. & Samulski, R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72, 2224–2232 (1998).
Gao, G.P. et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99, 11854–11859 (2002).
De, B.P. et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol. Ther. 13, 67–76 (2006).
Clark, K.R., Liu, X., McGrath, J.P. & Johnson, P.R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 10, 1031–1039 (1999).
Wu, M., Shanabrough, M., Leranth, C. & Alreja, M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J. Neurosci. 20, 3900–3908 (2000).
Petreanu, L., Mao, T., Sternson, S.M. & Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009).
Hull, C., Adesnik, H. & Scanziani, M. Neocortical disynaptic inhibition requires somatodendritic integration in interneurons. J. Neurosci. 29, 8991–8995 (2009).
Acknowledgements
We are grateful to Q.H. Ha, M. Ha and S. Keating for superb technical assistance and M. Debruyne for helpful comments. We are also indebted to B. Lowell and L. Vong (Beth Israel Deaconess Medical Center) for supplying us with the breeder pairs for developing our Vgat-IRES-cre and Vglut2-IRES-cre mouse lines. This work was supported by US National Institutes of Health grants NS073613, NS061863, NS062727, NS061841, NS085477, DA024763, MH103399 and HL095491 and by the G. Harold and Leila Y. Mathers Foundation.
Author information
Authors and Affiliations
Contributions
C.A., E.A., C.B.S., J.L. and P.M.F. designed the experiments. C.A. and L.F. carried out the experiments. C.A., C.E.B., E.A. and P.M.F. provided reagents and analytic tools. C.A., L.F., E.A. and P.M.F. analyzed the data. C.A., E.A., C.B.S. and P.M.F. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The extent of transduced neurons (that is, mCherry-positive somas) is shown for individual Vgat-IRES-cre mice that received bilateral injections of hM3Dq-AAV.
Rostral medullary regions containing hM3Dq transfected neurons are outlined for n = 13 mice included in the study, across 4 panels. hM3Dq-expressing neurons were located in the rostral medulla, near the medullo-pontine junction, lateral to the facial nerve, ventral to the locus coeruleus and vestibular complex and medial to the principal sensory and spinal trigeminal nuclei.
Supplementary Figure 2 hM3Dq receptor expression in PZ GABAergic neurons does not alter baseline (that is, in the absence of ligand (CNO)) sleep-wake.
Panels a, b & c show hourly sleep-wake amounts (± SEM) in Vgat-IRES-cre mice (n = 13) expressing hM3Dq receptor in GABAergic PZ neurons as compared with non-cre-expressing littermate mice (n = 12). Panels d, e & f show sleep-wake power bands (± SEM) (δ 0.4-4.3 Hz, θ 4.3-9.8, α 9.8-19.9 Hz, β + γ 19.9-59.8 Hz) from the first half of the dark (active) period in both groups of mice (n = 8 hM3Dq+ and 9 controls), and expressed in % of total power (0.4-59.8 Hz). Neither sleep-wake amounts nor the state-specific power bands differed between Vgat-ires-cre mice expressing hM3Dq in GABAergic PZ neurons and non-hM3Dq-expressing wild type littermate mice, indicating that expression of the (inactive) hM3Dq receptor has no impact on baseline SWS, wake, REM sleep or the respective state EEG spectral content.
Supplementary Figure 3 Administration of the hM3Dq receptor ligand, CNO, has no effect on the sleep-wake cycle of control mice, that is, non–Cre-expressing littermates.
Panels a, b & c show hourly sleep-wake amounts (± SEM) following injections of CNO (0.3 mg/kg, IP; 7PM) as compared with vehicle injection in control mice (n = 12). Panel d, e & f show sleep-wake power bands (± SEM) (δ 0.4-4.3 Hz, θ 4.3-9.8, α 9.8-19.9 Hz, β + γ 19.9-59.8 Hz) from the first three hours of the dark period in these mice (n = 7) following vehicle or CNO injections and expressed in percentage of baseline power band at the same time of the day (7PM-10PM). Neither sleep-wake amounts nor the state-specific power spectra differed following CNO and vehicle injections in control mice, indicating that CNO at the dosage used in this study is pharmacologically inert on sleep, wake and REM and associated EEG spectra. CNO: clozapine-N-oxide.
Supplementary Figure 4 Activation of PZ GABAergic neurons induces normal slow-wave sleep (SWS) during the subjective day (inactive period, a time of high sleep drive in mice).
SWS power spectra and frequency band expressed in % of total power. Panel a shows the SWS power spectrum during the 3 hrs post-injection period for vehicle injection as compared with the first hour post-injection period for CNO (0.3 mg/kg, IP; 7PM; n = 8 mice). Panel b shows the quantitative changes (± SEM) in power for the δ (0.4-4.3 Hz), θ (4.3-9.8 Hz), α (9.8-19.9 Hz) and β+ γ (19.9-59.8 Hz) frequency bands following CNO (0.3 mg/kg, IP; ZT12; n = 8) administration. CNO: clozapine-N-oxide.
Supplementary Figure 5 c-Fos expression in the cortex of PZ M3-expressing mice and littermate controls following acute CNO administration.
Mice expressing hM3Dq in PZ GABAergic neurons and non-cre-expressing littermates were sacrificed 2 hours after acute administration of CNO (0.3mg/kg, IP, 7PM, which is the beginning of the active period) and their brains were processed for c-Fos, a marker of cellular activation. (a-b) Mice expressing hM3Dq in PZ GABAergic neurons show dense PZ c-fos expression (b) following administration of the ligand, CNO, whereas (c-d) non-cre-expressing littermate mice show sparse c-fos PZ expression (d) following administration of CNO. (a) Consistent with the induction of behavioral sleep and electrographic SWS following CNO administration, very little c-Fos was observed in cortical regions of mice expressing hM3Dq in PZ GABAergic neurons, i.e., cellular cortex was quiescent. (c) By contrast, non-cre-expressing mice exhibited high c-Fos expression levels in cortical regions, i.e., neuronal activity. These cortical c-Fos findings are consistent with the behavioral and EEG changes observed following CNO administration in hM3Dq-expressing mice (high SWS, low wake) and non-cre-expressing littermates (low SWS, high wake). Scale bar = 100 μm. CNO: clozapine-N-oxide.
Supplementary Figure 6 Administration of CNO significantly increases waking and decreases SWS in mice expressing the hM4Di receptor in GABAergic PZ neurons.
Panels a, b & c show hourly sleep-wake amounts (± SEM) following injections of CNO (0.3 mg/kg, IP; 10AM) as compared with vehicle injection in mice with confirmed bilateral PZ transduction of hM4Di-AAV. Panel d, e & f show sleep-wake power bands (± SEM) (δ 0.4-4.3 Hz, θ 4.3-9.8, α 9.8-19.9 Hz, β + γ 19.9-59.8 Hz) from the first three hours after injection in these mice following vehicle or CNO injections and expressed in percentage of baseline power band at the same time of the day (10AM-1PM; a time of high ‘sleep drive’). Wake amounts are significantly increased and both SWS and REM sleep amounts were significantly decreased during 2 hr post CNO injection as compared with control injection. No statistically significant changes were seen in the EEG frequency bands between control and CNO condition. CNO: clozapine-N-oxide. * p < 0.05.
Supplementary Figure 7 PZ Vgat neurons expressing ChR2-mCherry respond to direct stimulation with blue-light pulses.
(a) After an injection of AAV-DIO-ChR2(H134R)-mCherry virus in Vgat-IRES-cre mice, PZVgat neurons express ChR2-mCherry (scale bar = 20 µm) (b) ChR2-mediated current (Vh = -60mV, in TTX 1 µM) evoked by a 300 ms blue-light pulse (blue bar). (c) Current-clamp (top traces) and voltage-clamp (bottom traces; Vh = -60mV) recordings of a ChR2-mCherry expressing PZVgat neuron following photostimulation at different frequencies (trains of ten 2 ms blue-light flashes, blue bars). The response to the last light flash of each stimulation paradigm is represented in the insert on the right. All recordings were conducted using a K-gluconate-based pipette solution.
Supplementary Figure 8 Schematic model of the principal findings described in this study.
When activated in vivo GABAergic PZ neurons potently and rapidly induce SWS and cortical SWA. Activation of GABAergic PZ neurons also results in the release of synaptic GABA (red arrow) onto BFmc-projecting PB neurons (green arrow). Moreover, activation of glutamatergic PB neurons results in the release of synaptic glutamate (blue arrow) onto cortically-projecting BF neurons. The net result is a dramatic reduction in ascending activating influences normally provided by the PB and BFmc to the cortex. This is reflected in the cortical EEG as a decrease in the fast frequencies that are characteristic of cortical activation/desynchronization and wakefulness and the appearance of slow waves and delta waves that are characteristic of cortical synchronization and SWS. BFmc: magnocellular basal forebrain; PB: parabrachial nucleus; PFC: prefrontal cortex; PZ: parafacial zone; SWS: slow-wave-sleep; SWA: slow-wave activity.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 4138 kb)
Supplementary Methods Checklist
(PDF 425 kb)
Rights and permissions
About this article
Cite this article
Anaclet, C., Ferrari, L., Arrigoni, E. et al. The GABAergic parafacial zone is a medullary slow wave sleep–promoting center. Nat Neurosci 17, 1217–1224 (2014). https://doi.org/10.1038/nn.3789
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3789
This article is cited by
-
Proteostasis failure exacerbates neuronal circuit dysfunction and sleep impairments in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Neuro-orchestration of sleep and wakefulness
Nature Neuroscience (2023)
-
Tfap2b acts in GABAergic neurons to control sleep in mice
Scientific Reports (2023)
-
Sleep apnea pathophysiology
Sleep and Breathing (2023)
-
Control of non-REM sleep by ventrolateral medulla glutamatergic neurons projecting to the preoptic area
Nature Communications (2022)