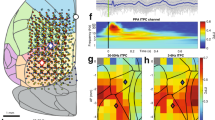
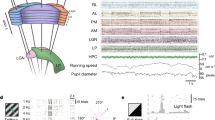
Abstract
Using millisecond-timescale voltage-sensitive dye imaging in lightly anesthetized or awake adult mice, we show that a palette of sensory-evoked and hemisphere-wide activity motifs are represented in spontaneous activity. These motifs can reflect multiple modes of sensory processing, including vision, audition and touch. We found similar cortical networks with direct cortical activation using channelrhodopsin-2. Regional analysis of activity spread indicated modality-specific sources, such as primary sensory areas, a common posterior-medial cortical sink where sensory activity was extinguished within the parietal association area and a secondary anterior medial sink within the cingulate and secondary motor cortices for visual stimuli. Correlation analysis between functional circuits and intracortical axonal projections indicated a common framework corresponding to long-range monosynaptic connections between cortical regions. Maps of intracortical monosynaptic structural connections predicted hemisphere-wide patterns of spontaneous and sensory-evoked depolarization. We suggest that an intracortical monosynaptic connectome shapes the ebb and flow of spontaneous cortical activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zhang, D. & Raichle, M.E. Disease and the brain's dark energy. Nat. Rev. Neurol. 6, 15–28 (2010).
Destexhe, A. & Contreras, D. Neuronal computations with stochastic network states. Science 314, 85–90 (2006).
Harris, K.D. & Thiele, A. Cortical state and attention. Nat. Rev. Neurosci. 12, 509–523 (2011).
Arieli, A., Sterkin, A., Grinvald, A. & Aertsen, A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273, 1868–1871 (1996).
Kenet, T., Bibitchkov, D., Tsodyks, M., Grinvald, A. & Arieli, A. Spontaneously emerging cortical representations of visual attributes. Nature 425, 954–956 (2003).
Ferezou, I. et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56, 907–923 (2007).
Waters, J. & Helmchen, F. Background synaptic activity is sparse in neocortex. J. Neurosci. 26, 8267–8277 (2006).
Luczak, A., Bartho, P. & Harris, K.D. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62, 413–425 (2009).
Han, F., Caporale, N. & Dan, Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron 60, 321–327 (2008).
Vincent, J.L. et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 (2007).
Leopold, D.A., Murayama, Y. & Logothetis, N.K. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex 13, 422–433 (2003).
Lu, H. et al. Rat brains also have a default mode network. Proc. Natl. Acad. Sci. USA 109, 3979–3984 (2012).
de Pasquale, F. et al. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron 74, 753–764 (2012).
Hipp, J.F., Hawellek, D.J., Corbetta, M., Siegel, M. & Engel, A.K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 15, 884–890 (2012).
Wedeen, V.J. et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41, 1267–1277 (2008).
Frostig, R.D., Xiong, Y., Chen-Bee, C.H., Kvasnak, E. & Stehberg, J. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J. Neurosci. 28, 13274–13284 (2008).
White, B.R. et al. Imaging of functional connectivity in the mouse brain. PLoS ONE 6, e16322 (2011).
Grinvald, A. & Hildesheim, R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5, 874–885 (2004).
Mohajerani, M.H., McVea, D.A., Fingas, M. & Murphy, T.H. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J. Neurosci. 30, 3745–3751 (2010).
Allen Institute for Brain Science. Allen Mouse Brain Connectivity Atlas. Allen Brain Atlas Data Portal 〈http://connectivity.brain-map.org/〉 (2012).
Kleinfeld, D. & Delaney, K.R. Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes. J. Comp. Neurol. 375, 89–108 (1996).
Lim, D.H. et al. In vivo large-scale cortical mapping using channelrhodopsin-2 stimulation in transgenic mice reveals asymmetric and reciprocal relationships between cortical areas. Front. Neural Circuits 6, 11 (2012).
Mao, T. et al. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72, 111–123 (2011).
Grandy, T.H., Greenfield, S.A. & Devonshire, I.M. An evaluation of in vivo voltage-sensitive dyes: pharmacological side effects and signal-to-noise ratios after effective removal of brain-pulsation artifacts. J. Neurophysiol. 108, 2931–2945 (2012).
Akemann, W. et al. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 108, 2323–2337 (2012).
Liu, C. Beyond Pixels: Exploring New Representations and Applications for Motion Analysis. PhD thesis, Massachusetts Institute of Technology (2009).
Takagaki, K., Zhang, C., Wu, J.Y. & Ohl, F.W. Flow detection of propagating waves with temporospatial correlation of activity. J. Neurosci. Methods 200, 207–218 (2011).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Poulet, J.F. & Petersen, C.C. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008).
Haider, B., Hausser, M. & Carandini, M. Inhibition dominates sensory responses in the awake cortex. Nature 493, 97–100 (2013).
Polack, P.O. & Contreras, D. Long-range parallel processing and local recurrent activity in the visual cortex of the mouse. J. Neurosci. 32, 11120–11131 (2012).
Huang, X. et al. Spiral wave dynamics in neocortex. Neuron 68, 978–990 (2010).
Sato, T.K., Nauhaus, I. & Carandini, M. Traveling waves in visual cortex. Neuron 75, 218–229 (2012).
Niell, C.M. & Stryker, M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Wilson, M.A. & McNaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994).
Euston, D.R., Tatsuno, M. & McNaughton, B.L. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science 318, 1147–1150 (2007).
Douglas, R.J. & Martin, K.A. Mapping the matrix: the ways of neocortex. Neuron 56, 226–238 (2007).
Reid, R.C. From functional architecture to functional connectomics. Neuron 75, 209–217 (2012).
Shmuel, A. & Leopold, D.A. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum. Brain Mapp. 29, 751–761 (2008).
Grienberger, C. et al. Sound-evoked network calcium transients in mouse auditory cortex in vivo. J. Physiol. (Lond.) 590, 899–918 (2012).
Buzsaki, G. et al. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 8, 4007–4026 (1988).
Beier, K.T. et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc. Natl. Acad. Sci. USA 108, 15414–15419 (2011).
Osakada, F. et al. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71, 617–631 (2011).
Cardin, J.A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Tamamaki, N. & Tomioka, R. Long-range GABAergic connections distributed throughout the neocortex and their possible function. Front. Neurosci. 4, 202 (2010).
Alivisatos, A.P. et al. The brain activity map project and the challenge of functional connectomics. Neuron 74, 970–974 (2012).
Bohland, J.W. et al. A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLoS Comput. Biol. 5, e1000334 (2009).
Li, A. et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science 330, 1404–1408 (2010).
Kleinfeld, D. et al. Large-scale automated histology in the pursuit of connectomes. J. Neurosci. 31, 16125–16138 (2011).
Azin, M., Guggenmos, D.J., Barbay, S., Nudo, R.J. & Mohseni, P. A miniaturized system for spike-triggered intracortical microstimulation in an ambulatory rat. IEEE Trans. Biomed. Eng. 58, 2589–2597 (2011).
Shoham, D. et al. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron 24, 791–802 (1999).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Elsevier Academic Press, Amsterdam, Boston, 2004).
Acknowledgements
This work was supported by Canadian Institutes of Health Research (CIHR) Operating Grant MOP-12675 (T.H.M.), a Human Frontier Science Program grant (T.H.M.), Michael Smith Foundation for Health Research postdoctoral fellowships (M.H.M. and A.W.C.), a CIHR Operating Grant (Y.T.W.), Heart and Stroke Foundation of Canada postdoctoral fellowships (M.H.M. and A.W.C.) and a CIHR Focus on Stroke postdoctoral fellowship (M.H.M.). We thank the Allen Institute for Brain Science for providing a database of axonal projections. We thank P. Wang and C. Jiang for surgical assistance.
Author information
Authors and Affiliations
Contributions
M.H.M. and T.H.M. designed the study. M.H.M., A.W.C., D.A.M. and J.L. performed the experiments. M.H.M., A.W.C., M.M., J.L., Y.T.W. and M.R. analyzed the data. M.H.M., R.L., J.L. and J.D.B. participated in processing the Allen Mouse Brain Connectivity data. M.H.M., A.W.C. and T.H.M. wrote the manuscript, which all authors commented on and edited. T.H.M. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-10 (PDF 3997 kb)
Unique regional patterns of cortical depolarization after different forms of sensory stimulation.
Tone (1ms, 25 KHz), C2 whisker (1ms, tactile stimulation using piezo), visual (1ms, green and blue LED), forelimb (1ms, 1mA), and hindlimb (1ms, 1mA) evoked VSD response were imaged in an isoflurane anesthetized mouse. The imaging area included: primary (motor, somatosensory, visual, Auditory), secondary (motor, visual, somatosensory) and association (retrosplenial, cingulate) cortices. (AVI 2015 kb)
Spontaneous cortical activity in an anesthetized mouse.
Spontaneous cortical activity across the right hemisphere imaged in an isoflurane (0.5%) anesthetized mouse. The VSD signal of spontaneous cortical activity was filtered (0.1-6 Hz) using a zero-phase Chebyshev bandpass filter. (AVI 19426 kb)
Optical flow measurement of C2 whisker-evoked VSD response.
Whisker stimulation-evoked VSD activation (average of 20 trials). Left, black arrows indicate the direction of velocity of VSD signal spread. Relative magnitude of velocity is indicated by arrows size. Right, for response in left, measurements of absolute velocity are represented in pseudocolor. Streamlines indicate local direction of velocity flow. (AVI 2236 kb)
Time evolution of seed-pixel correlation map.
Spontaneous cortical activity in an isoflurane (0.5%) anesthetized mouse alongside the cumulative seed-pixel based correlation maps for the forelimb cortex. (AVI 12135 kb)
41593_2013_BFnn3499_MOESM17_ESM.avi
The spatiotemporal dynamics of visual-evoked VSD response under conditions of isoflurane anesthesia or in a quiet awake state. (AVI 629 kb)
Example of averaged hindlimb-evoked VSD response followed by an example of a hindlimb-evoked-like event within spontaneous activity.
The hindlimb evoked response is the average of 10 trials of contralateral (left) hindlimb stimulation. Episodes of spontaneous activity were detected by cross-correlation analysis of spontaneous activity and a 3-frame hindlimb response template that exceeded threshold criteria (see Methods). The detected hindlimb-evoked-like spontaneous events were then aligned by the start of the template and averaged to make the movie. (AVI 1616 kb)
Example of averaged whisker-evoked VSD response followed by an example of a whisker-evoked-like event within spontaneous activity.
The whisker evoked response is the average of 10 trials of contralateral (left) whisker stimulation. Episodes of spontaneous activity were detected by cross-correlation analysis of spontaneous activity and a 3-frame whisker response template that exceeded threshold criteria (see Methods). The detected whisker-evoked-like spontaneous events were then aligned by the start of the template and averaged to make the movie. (AVI 1680 kb)
Rights and permissions
About this article
Cite this article
Mohajerani, M., Chan, A., Mohsenvand, M. et al. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat Neurosci 16, 1426–1435 (2013). https://doi.org/10.1038/nn.3499
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3499
This article is cited by
-
Rapid fluctuations in functional connectivity of cortical networks encode spontaneous behavior
Nature Neuroscience (2024)
-
Cortical glutamatergic projection neuron types contribute to distinct functional subnetworks
Nature Neuroscience (2023)
-
Selective effects of acute and chronic stress on slow and alpha-theta cortical functional connectivity and reversal with subanesthetic ketamine
Neuropsychopharmacology (2023)
-
Complexity of cortical wave patterns of the wake mouse cortex
Nature Communications (2023)
-
Spatio-temporal activation patterns of neuronal population evoked by optostimulation and the comparison to electrical microstimulation
Scientific Reports (2023)