Abstract
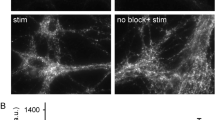
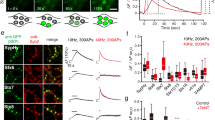
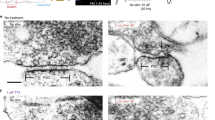
Although clathrin-mediated endocytosis is thought to be the predominant mechanism of synaptic vesicle recycling, it seems to be too slow for fast recycling. Therefore, it was suggested that a presorted and preassembled pool of synaptic vesicle proteins on the presynaptic membrane might support a first wave of fast clathrin-mediated endocytosis. In this study we monitored the temporal dynamics of such a 'readily retrievable pool' of synaptic vesicle proteins in rat hippocampal neurons using a new type of probe. By applying cypHer5E, a new cyanine dye–based pH-sensitive exogenous marker, coupled to antibodies to luminal domains of synaptic vesicle proteins, we could reliably monitor synaptic vesicle recycling and demonstrate the preferential recruitment of a surface pool of synaptic vesicle proteins upon stimulated endocytosis. By using fluorescence nanoscopy of surface-labeled synaptotagmin 1, we could resolve the spatial distribution of the surface pool at the periactive zone in hippocampal boutons, which represent putative sites of endocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Heuser, J.E. & Reese, T.S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57, 315–344 (1973).
Granseth, B., Odermatt, B., Royle, S.J. & Lagnado, L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773–786 (2006).
Mueller, V.J., Wienisch, M., Nehring, R.B. & Klingauf, J. Monitoring clathrin-mediated endocytosis during synaptic activity. J. Neurosci. 24, 2004–2012 (2004).
Wu, L.G., Ryan, T.A. & Lagnado, L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J. Neurosci. 27, 11793–11802 (2007).
He, L., Wu, X.S., Mohan, R. & Wu, L.G. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature 444, 102–105 (2006).
Zhang, Q., Li, Y. & Tsien, R.W. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 323, 1448–1453 (2009).
Ceccarelli, B., Hurlbut, W.P. & Mauro, A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J. Cell Biol. 57, 499–524 (1973).
Gandhi, S.P. & Stevens, C.F. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423, 607–613 (2003).
Koenig, J.H., Yamaoka, K. & Ikeda, K. Omega images at the active zone may be endocytotic rather than exocytotic: implications for the vesicle hypothesis of transmitter release. Proc. Natl. Acad. Sci. USA 95, 12677–12682 (1998).
Miesenböck, G., De Angelis, D.A. & Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998).
Fernández-Alfonso, T., Kwan, R. & Ryan, T.A. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron 51, 179–186 (2006).
Dittman, J.S. & Kaplan, J.M. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc. Natl. Acad. Sci. USA 103, 11399–11404 (2006).
Wienisch, M. & Klingauf, J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat. Neurosci. 9, 1019–1027 (2006).
Teng, H., Cole, J.C., Roberts, R.L. & Wilkinson, R.S. Endocytic active zones: hot spots for endocytosis in vertebrate neuromuscular terminals. J. Neurosci. 19, 4855–4866 (1999).
Teng, H. & Wilkinson, R.S. Clathrin-mediated endocytosis near active zones in snake motor boutons. J. Neurosci. 20, 7986–7993 (2000).
Taubenblatt, P., Dedieu, J.C., Gulik-Krzywicki, T. & Morel, N. VAMP (synaptobrevin) is present in the plasma membrane of nerve terminals. J. Cell Sci. 112, 3559–3567 (1999).
Willig, K.I., Rizzoli, S.O., Westphal, V., Jahn, R. & Hell, S.W. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature 440, 935–939 (2006).
Miller, T.M. & Heuser, J.E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J. Cell Biol. 98, 685–698 (1984).
Balaji, J. & Ryan, T.A. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc. Natl. Acad. Sci. USA 104, 20576–20581 (2007).
Adie, E.J. et al. CypHer 5: a generic approach for measuring the activation and trafficking of G protein-coupled receptors in live cells. Assay Drug Dev. Technol. 1, 251–259 (2003).
Martens, H. et al. Unique luminal localization of VGAT-C terminus allows for selective labeling of active cortical GABAergic synapses. J. Neurosci. 28, 13125–13131 (2008).
Hüve, J., Wesselmann, R., Kahms, M. & Peters, R. 4Pi microscopy of the nuclear pore complex. Biophys. J. 95, 877–885 (2008).
Kano, H., Jakobs, S., Nagorni, M. & Hell, S.W. Dual-color 4Pi-confocal microscopy with 3D-resolution in the 100 nm range. Ultramicroscopy 90, 207–213 (2001).
Schmidt, R. et al. Spherical nanosized focal spot unravels the interior of cells. Nat. Methods 5, 539–544 (2008).
Roos, J. & Kelly, R.B. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr. Biol. 9, 1411–1414 (1999).
Shupliakov, O. et al. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc. Natl. Acad. Sci. USA 99, 14476–14481 (2002).
Gundelfinger, E.D., Kessels, M.M. & Qualmann, B. Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 4, 127–139 (2003).
Sankaranarayanan, S., De Angelis, D., Rothman, J.E. & Ryan, T.A. The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 79, 2199–2208 (2000).
Sankaranarayanan, S. & Ryan, T.A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2, 197–204 (2000).
Fernández-Alfonso, T. & Ryan, T.A. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron 41, 943–953 (2004).
Schikorski, T. & Stevens, C.F. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 17, 5858–5867 (1997).
Murthy, V.N. & Stevens, C.F. Reversal of synaptic vesicle docking at central synapses. Nat. Neurosci. 2, 503–507 (1999).
Schikorski, T. & Stevens, C.F. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 4, 391–395 (2001).
Tabares, L. et al. Monitoring synaptic function at the neuromuscular junction of a mouse expressing synaptopHluorin. J. Neurosci. 27, 5422–5430 (2007).
Takamori, S. et al. Molecular anatomy of a trafficking organelle. Cell 127, 831–846 (2006).
Vanden Berghe, P. & Klingauf, J. Synaptic vesicles in rat hippocampal boutons recycle to different pools in a use-dependent fashion. J. Physiol. (Lond.) 572, 707–720 (2006).
Staras, K. et al. A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron 66, 37–44 (2010).
Fredj, N.B. & Burrone, J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat. Neurosci. 12, 751–758 (2009).
Groemer, T.W. & Klingauf, J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat. Neurosci. 10, 145–147 (2007).
Sara, Y., Virmani, T., Deak, F., Liu, X. & Kavalali, E.T. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45, 563–573 (2005).
Hua, Y., Sinha, R., Martineau, M., Kahms, M. & Klingauf, J. A common origin of synaptic vesicles undergoing evoked and spontaneous fusion. Nat. Neurosci. 13, 1451–1453 (2010).
Brodin, L. & Shupliakov, O. Giant reticulospinal synapse in lamprey: molecular links between active and periactive zones. Cell Tissue Res. 326, 301–310 (2006).
Kim, S.H. & Ryan, T.A. Synaptic vesicle recycling at CNS synapses without AP-2. J. Neurosci. 29, 3865–3874 (2009).
Glyvuk, N. et al. AP-1/σ1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 29, 1318–1330 (2010).
Hosoi, N., Holt, M. & Sakaba, T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron 63, 216–229 (2009).
Wu, X.S. et al. Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat. Neurosci. 12, 1003–1010 (2009).
Yao, C.K. et al. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell 138, 947–960 (2009).
Goslin, K. & Banker, G. Rat hippocampal neurons in low-density culture. in Culturing Nerve Cells 1st edn. (eds. Banker, G. & Goslin, K.) 251–281 (MIT Press, Cambridge, Massachusetts, USA, 1991).
Staudt, T., Lang, M.C., Medda, R., Engelhardt, J. & Hell, S.W. 2,2′-thiodiethanol: a new water soluble mounting medium for high resolution optical microscopy. Microsc. Res. Tech. 70, 1–9 (2007).
Richardson, W.H. Bayesian-based iterative method of image restoration. J. Opt. Soc. Am. 62, 55–59 (1972).
Acknowledgements
We thank M. Pilot and I. Herfort for the preparation of primary cell cultures of hippocampal neurons and E. Neher and R.H. Chow for critical reading of the manuscript. We acknowledge D. Boening and M. Martineau for experimental support and we are grateful to K. Kolmakov and V. Belov (Max Planck Institute for Biophysical Chemistry, Goettingen) for providing us with the new fluorescent dye KK114. We thank G. Miesenböck (Oxford University) for providing superecliptic spH. We would also like to thank M. Hoon and N. Glyvuk for suggestions. This work was supported by grants from the European Science Foundation/Deutsche Forschungsgemeinschaft (DFG) (EUROMEMBRANE programme, EuroSynapse CRP FP-020 to J.K.) as well as from the DFG (Kl 1334/1-1 to C.S.T. and J.K., SFB 944 to J.K., CMPB to A.E. and S.W.H., and SFB755 to A.E. and S.W.H.). Y.H. is supported by a stipend from the Max-Planck Foundation and R. Sinha is supported by a stipend from the International Max Planck Research School in Neurosciences at the University of Goettingen.
Author information
Authors and Affiliations
Contributions
Y.H., R. Sinha and C.S.T. conducted the majority of the experiments. J.H. collected and analyzed the 4Pi microscopy data. IsoSTED microscopy and analysis was performed by R. Schmidt and A.E. in the department of S.W.H. H.M. synthesized the cypHer-conjugated antibodies. J.K. conceptualized the project and together with Y.H. and R. Sinha designed the experiments. R. Sinha and J.K. wrote the paper with the help of Y.H., C.S.T. and R. Schmidt. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 567 kb)
Rights and permissions
About this article
Cite this article
Hua, Y., Sinha, R., Thiel, C. et al. A readily retrievable pool of synaptic vesicles. Nat Neurosci 14, 833–839 (2011). https://doi.org/10.1038/nn.2838
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2838
This article is cited by
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Organic fluorescent probes for live-cell super-resolution imaging
Frontiers of Optoelectronics (2023)
-
Extracellular matrix remodeling through endocytosis and resurfacing of Tenascin-R
Nature Communications (2021)
-
Aβ1-16 controls synaptic vesicle pools at excitatory synapses via cholinergic modulation of synapsin phosphorylation
Cellular and Molecular Life Sciences (2021)
-
Imaging endocytic vesicle formation at high spatial and temporal resolutions with the pulsed-pH protocol
Nature Protocols (2020)