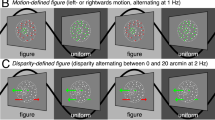
Abstract
Processing of visual information is both parallel and hierarchical, with each visual area richly interconnected with other visual areas. An example of the parallel architecture of the primate visual system is the existence of two principal pathways providing input to the middle temporal visual area (MT): namely, a direct projection from striate cortex (V1), and a set of indirect projections that also originate in V1 but then relay through V2 and V3. Here we have reversibly inactivated the indirect pathways while recording from MT neurons and measuring eye movements in alert monkeys, a procedure that has enabled us to assess whether the two different input pathways are redundant or whether they carry different kinds of information. We find that this inactivation causes a disproportionate degradation of binocular disparity tuning relative to direction tuning in MT neurons, suggesting that the indirect pathways are important in the recovery of depth in three-dimensional scenes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Broca, P.P. Localisation des fonctions cérébrales: Siège du langage articulé. Bull. De La Société D'Anthropologie Tome IV 200–208 (1863).
Fritsch, G. & Hitzig, E. Uber die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol. wissenschaftl. Med. 37, 300–332 (1870).
Felleman, D.J. & Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Maunsell, J.H. & Van Essen, D.C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J. Neurophysiol. 49, 1127–1147 (1983).
Shipp, S. & Zeki, S. The organization of connections between areas V5 and V2 in macaque monkey visual cortex. Eur. J. Neurosci. 1, 333–354 (1989).
Sincich, L.C. & Horton, J.C. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. J. Neurosci. 23, 5684–5692 (2003).
Yabuta, N.H., Sawatari, A. & Callaway, E.M. Two functional channels from primary visual cortex to dorsal visual cortical areas. Science 292, 297–300 (2001).
Movshon, J.A. & Newsome, W.T. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J. Neurosci. 16, 7733–7741 (1996).
DeYoe, E.A. & Van Essen, D.C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature 317, 58–61 (1985).
Peterhans, E. & von der Heydt, R. Functional organization of area V2 in the alert macaque. Eur. J. Neurosci. 5, 509–524 (1993).
Hubel, D.H. & Livingstone, M.S. Segregation of form, color and stereopsis in primate area 18. J. Neurosci. 7, 3378–3415 (1987).
Orban, G.A., Kennedy, H. & Bullier, J. Velocity sensitivity and direction selectivity of neurons in areas V1 and V2 of the monkey: influence of eccentricity. J. Neurophysiol. 56, 462–480 (1986).
Cumming, B.G. & DeAngelis, G.C. The physiology of stereopsis. Annu. Rev. Neurosci. 24, 203–238 (2001).
Girard, P., Salin, P.A. & Bullier, J. Response selectivity of neurons in area MT of the macaque monkey during reversible inactivation of area V1. J. Neurophysiol. 67, 1437–1446 (1992).
Gattass, R., Gross, C.G. & Sandell, J.H. Visual topography of V2 in the macaque. J. Comp. Neurol. 201, 519–539 (1981).
Gattass, R., Sousa, A.P. & Gross, C.G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 8, 1831–1845 (1988).
Prince, S.J., Cumming, B.G. & Parker, A.J. Range and mechanism of encoding of horizontal disparity in macaque V1. J. Neurophysiol. 87, 209–221 (2002).
DeAngelis, G.C. & Uka, T. Coding of horizontal disparity and velocity by MT neurons in the alert macaque. J. Neurophysiol. 89, 1094–1111 (2003).
Zar, J.H. Biostatistical Analysis, 380–382 (Prentice Hall, Upper Saddle River, New Jersey, 1999).
Tsao, D.Y. et al. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron 39, 555–568 (2003).
Born, R.T. & Bradley, D.C. Structure and function of visual area MT. Annu. Rev. Neurosci. 28, 157–189 (2005).
Komatsu, H. & Wurtz, R.H. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J. Neurophysiol. 60, 580–603 (1988).
Newsome, W.T., Wurtz, R.H., Dursteler, M.R. & Mikami, A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J. Neurosci. 5, 825–840 (1985).
Dursteler, M.R. & Wurtz, R.H. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J. Neurophysiol. 60, 940–965 (1988).
Inoue, Y., Takemura, A., Kawano, K., Kitama, T. & Miles, F.A. Dependence of short-latency ocular following and associated activity in the medial superior temporal area (MST) on ocular vergence. Exp. Brain Res. 121, 135–144 (1998).
Busettini, C., Miles, F.A. & Krauzlis, R.J. Short-latency disparity vergence responses and their dependence on a prior saccadic eye movement. J. Neurophysiol. 75, 1392–1410 (1996).
Takemura, A., Murata, Y., Kawano, K. & Miles, F.A. Deficits in short-latency tracking eye movements after chemical lesions in monkey cortical areas MT and MST. J. Neurosci. 27, 529–541 (2007).
Hupé, J.M., James, A.C., Girard, P. & Bullier, J. Response modulations by static texture surround in area V1 of the macaque monkey do not depend on feedback connections from V2. J. Neurophysiol. 85, 146–163 (2001).
Sandell, J.H. & Schiller, P.H. Effect of cooling area 18 on striate cortex cells in the squirrel monkey. J. Neurophysiol. 48, 38–48 (1982).
Prince, S.J., Pointon, A.D., Cumming, B.G. & Parker, A.J. Quantitative analysis of the responses of V1 neurons to horizontal disparity in dynamic random-dot stereograms. J. Neurophysiol. 87, 191–208 (2002).
Qian, N. & Andersen, R.A. A physiological model for motion-stereo integration and a unified explanation of Pulfrich-like phenomena. Vision Res. 37, 1683–1698 (1997).
Anzai, A., Ohzawa, I. & Freeman, R.D. Joint-encoding of motion and depth by visual cortical neurons: neural basis of the Pulfrich effect. Nat. Neurosci. 4, 513–518 (2001).
Pack, C.C., Born, R.T. & Livingstone, M.S. Two-dimensional substructure of stereo and motion interactions in macaque visual cortex. Neuron 37, 525–535 (2003).
Bradley, D.C., Qian, N. & Andersen, R.A. Integration of motion and stereopsis in middle temporal cortical area of macaques. Nature 373, 609–611 (1995).
Bradley, D.C., Chang, G.C. & Andersen, R.A. Encoding of three-dimensional structure-from-motion by primate area MT neurons. Nature 392, 714–717 (1998).
Dodd, J.V., Krug, K., Cumming, B.G. & Parker, A.J. Perceptually bistable three-dimensional figures evoke high choice probabilities in cortical area MT. J. Neurosci. 21, 4809–4821 (2001).
Grossberg, S. The complementary brain: unifying brain dynamics and modularity. Trends Cogn. Sci. 4, 233–246 (2000).
Berzhanskaya, J., Grossberg, S. & Mingolla, E. Laminar cortical dynamics of visual form and motion interactions during coherent object motion perception. Spat. Vis. 20, 337–395 (2007).
Pack, C.C., Conway, B.R., Born, R.T. & Livingstone, M.S. Spatiotemporal structure of nonlinear subunits in macaque visual cortex. J. Neurosci. 26, 893–907 (2006).
Churchland, M.M., Priebe, N.J. & Lisberger, S.G. Comparison of the spatial limits on direction selectivity in visual areas MT and V1. J. Neurophysiol. 93, 1235–1245 (2005).
Gattass, R., Gross, C.G. & Sandell, J.H. Visual topography of V2 in the macaque. J. Comp. Neurol. 201, 519–539 (1981).
Gattass, R., Sousa, A.P. & Gross, C.G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 8, 1831–1845 (1988).
Albright, T.D., Desimone, R. & Gross, C.G. Columnar organization of directionally selective cells in visual area MT of the macaque. J. Neurophysiol. 51, 16–31 (1984).
DeAngelis, G.C. & Newsome, W.T. Organization of disparity-selective neurons in macaque area MT. J. Neurosci. 19, 1398–1415 (1999).
Chen, G., Lu, H.D. & Roe, A.W. Functional architecture of macaque cortical area V2 for depth surfaces revealed by optical imaging. Soc. Neurosci. Abst. 114.6 (2006).
Ohki, K. et al. Highly ordered arrangement of single neurons in orientation pinwheels. Nature 442, 925–928 (2006).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Lomber, S.G., Payne, B.R. & Horel, J.A. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J. Neurosci. Methods 86, 179–194 (1999).
Busettini, C., Masson, G.S. & Miles, F.A. A role for stereoscopic depth cues in the rapid visual stabilization of the eyes. Nature 380, 342–345 (1996).
Takemura, A., Inoue, Y., Kawano, K., Quaia, C. & Miles, F.A. Single-unit activity in cortical area MST associated with disparity-vergence eye movements: evidence for population coding. J. Neurophysiol. 85, 2245–2266 (2001).
Acknowledgements
We thank P. Hendrickson and A. Zaharia for technical assistance, and J.H.R. Maunsell, J.A. Assad, M. Livingstone and N. Price for comments on the manuscript. This work was supported by a grant to C.R.P. from the National Institutes of Neurological Disorders and Stroke (F31NS052926), a grant to S.G.L. from the Natural Sciences and Engineering Research Council of Canada (327442), an R01 grant to R.T.B. (EY11379), a Vision Core Grant (EY12196) and a gift from R. Brandon Fradd.
Author information
Authors and Affiliations
Contributions
R.T.B. conceived the initial inactivation project. C.R.P. performed the experiments and developed the project along with R.T.B. S.G.L. fabricated the cryoloops and, along with R.T.B., implanted them in all monkeys. C.R.P. and R.T.B. wrote the manuscript, and all authors participated in its editing.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 1201 kb)
Rights and permissions
About this article
Cite this article
Ponce, C., Lomber, S. & Born, R. Integrating motion and depth via parallel pathways. Nat Neurosci 11, 216–223 (2008). https://doi.org/10.1038/nn2039
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2039
This article is cited by
-
Anatomical and functional connectomes underlying hierarchical visual processing in mouse visual system
Brain Structure and Function (2022)
-
Neurophysiological considerations for visual implants
Brain Structure and Function (2022)
-
Visual perception and memory systems: from cortex to medial temporal lobe
Cellular and Molecular Life Sciences (2011)
-
Parallel processing strategies of the primate visual system
Nature Reviews Neuroscience (2009)
-
Disparity- and velocity-based signals for three-dimensional motion perception in human MT+
Nature Neuroscience (2009)