Abstract
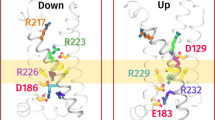
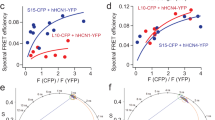
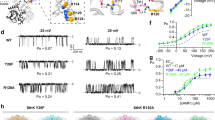
In the voltage-sensing phosphatase Ci-VSP, a voltage-sensing domain (VSD) controls a lipid phosphatase domain (PD). The mechanism by which the domains are allosterically coupled is not well understood. Using an in vivo assay, we found that the interdomain linker that connects the VSD to the PD is essential for coupling the full-length protein. Biochemical assays showed that the linker is also needed for activity in the isolated PD. We also identified a late step of VSD motion in the full-length protein that depends on the linker. Notably, we found that this VSD motion requires PI(4,5)P2, a substrate of Ci-VSP. These results suggest that the voltage-driven motion of the VSD turns the enzyme on by rearranging the linker into an activated conformation, and that this activated conformation is stabilized by PI(4,5)P2. We propose that Ci-VSP activity is self-limited because its decrease of PI(4,5)P2 levels decouples the VSD from the enzyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
17 September 2010
After publication, the authors found second-site mutations within two of the Ci-VSP constructs. The R253Q G214C and R254Q G214C constructs have been remade without the additional mutations and the data recollected. The data originally presented in Figure 1, Supplementary Figures 5 and 6 and Supplementary Table 1 have been updated to reflect the minor changes in the magnitude of the constructs’ effects. The changes do not affect the conclusions of the study. The changes have been made in the HTML and PDF versions of the article.
References
Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K. & Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243 (2005).
Okamura, Y., Murata, Y. & Iwasaki, H. Voltage-sensing phosphatase: actions and potentials. J. Physiol. (Lond.) 587, 513–520 (2009).
Worby, C.A. & Dixon, J.E. Phosphoinositide phosphatases: emerging roles as voltage sensors? Mol. Interv. 5, 274–277 (2005).
Lu, Z., Klem, A.M. & Ramu, Y. Ion conduction pore is conserved among potassium channels. Nature 413, 809–813 (2001).
Lu, Z., Klem, A.M. & Ramu, Y. Coupling between voltage sensors and activation gate in voltage-gated K+ channels. J. Gen. Physiol. 120, 663–676 (2002).
Long, S.B., Campbell, E.B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Murata, Y. & Okamura, Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J. Physiol. (Lond.) 583, 875–889 (2007).
Iwasaki, H. et al. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 105, 7970–7975 (2008).
Halaszovich, C.R., Schreiber, D.N. & Oliver, D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J. Biol. Chem. 284, 2106–2113 (2009).
Maehama, T., Taylor, G.S. & Dixon, J.E. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu. Rev. Biochem. 70, 247–279 (2001).
Iijima, M., Huang, Y.E., Luo, H.R., Vazquez, F. & Devreotes, P.N. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J. Biol. Chem. 279, 16606–16613 (2004).
Walker, S.M., Leslie, N.R., Perera, N.M., Batty, I.H. & Downes, C.P. The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem. J. 379, 301–307 (2004).
Vazquez, F. et al. Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc. Natl. Acad. Sci. USA 103, 3633–3638 (2006).
Rahdar, M. et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc. Natl. Acad. Sci. USA 106, 480–485 (2009).
Villalba-Galea, C.A., Miceli, F., Taglialatela, M. & Bezanilla, F. Coupling between the voltage-sensing and phosphatase domains of Ci-VSP. J. Gen. Physiol. 134, 5–14 (2009).
Mannuzzu, L.M., Moronne, M.M. & Isacoff, E.Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science 271, 213–216 (1996).
Kohout, S.C., Ulbrich, M.H., Bell, S.C. & Isacoff, E.Y. Subunit organization and functional transitions in Ci-VSP. Nat. Struct. Mol. Biol. 15, 106–108 (2008).
Hossain, M.I. et al. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J. Biol. Chem. 283, 18248–18259 (2008).
Villalba-Galea, C.A., Sandtner, W., Starace, D.M. & Bezanilla, F. S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. USA 105, 17600–17607 (2008).
Steck, P.A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 (1997).
Duerr, E.M. et al. PTEN mutations in gliomas and glioneuronal tumors. Oncogene 16, 2259–2264 (1998).
Gronbaek, K., Zeuthen, J., Guldberg, P., Ralfkiaer, E. & Hou-Jensen, K. Alterations of the MMAC1/PTEN gene in lymphoid malignancies. Blood 91, 4388–4390 (1998).
Downes, C.P. et al. Acute regulation of the tumour suppressor phosphatase, PTEN, by anionic lipids and reactive oxygen species. Biochem. Soc. Trans. 32, 338–342 (2004).
Xiao, Y. et al. PTEN catalysis of phospholipid dephosphorylation reaction follows a two-step mechanism in which the conserved aspartate-92 does not function as the general acid–mechanistic analysis of a familial Cowden disease-associated PTEN mutation. Cell. Signal. 19, 1434–1445 (2007).
Flint, A.J., Tiganis, T., Barford, D. & Tonks, N.K. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94, 1680–1685 (1997).
Julius, D., MacDermott, A.B., Axel, R. & Jessell, T.M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science 241, 558–564 (1988).
Suh, B.C., Inoue, T., Meyer, T. & Hille, B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 314, 1454–1457 (2006).
Varnai, P., Thyagarajan, B., Rohacs, T. & Balla, T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175, 377–382 (2006).
Lukacs, V. et al. Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080 (2007).
Campbell, R.B., Liu, F. & Ross, A.H. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 278, 33617–33620 (2003).
Redfern, R.E. et al. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry 47, 2162–2171 (2008).
Borah, H.N., Boruah, R.C. & Sandhu, J.S. Microwave-induced one-pot synthesis of N-carboxyalkyl maleimides and phthalimides. J. Chem. Res. (S) 272–273 (1998).
Acknowledgements
This work was supported by grants R01NS035549 (E.Y.I.), U24NS57631 (E.Y.I.) and R01DC007664 (D.L.M.) from the US National Institutes of Health and by an American Heart Association Established Investigator Award (D.L.M.). We thank Y. Okamura (Japanese National Institute for Physiological Sciences) for kindly providing the Ci-VSP cDNA, T. Meyer (Stanford University) for kindly providing the LDR and CFInp cDNA, E. Reuveny (Weizmann Institute of Science) for providing the IRK1 construct, S. Nakanishi (Osaka Bioscience Institute) for providing the mGluR1α receptor, J. Groves and P. Nair (University of California, Berkeley) for access to and instructions on use of the lipid extruder, and H. Janovjak, K. Nakajo, E. Peled and F. Tombola for helpful discussions.
Author information
Authors and Affiliations
Contributions
S.C.K. designed, conducted and analyzed the VCF experiments and the confocal experiment, conducted and analyzed the malachite green assay and the CD spectroscopy, and wrote the paper. S.C.B. designed, conducted and analyzed the in vivo activity assay and edited the paper. L.L. expressed and purified the cytosolic domain proteins and edited the paper. Q.X. helped design the malachite green assay and expressed and purified the cytosolic domain proteins. D.L.M. designed and initiated the CD spectroscopy and edited the paper. E.Y.I. designed the VCF experiments and the in vivo activity assay and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Tables 1–3, Supplementary Methods and Supplementary Results (PDF 237 kb)
Rights and permissions
About this article
Cite this article
Kohout, S., Bell, S., Liu, L. et al. Electrochemical coupling in the voltage-dependent phosphatase Ci-VSP. Nat Chem Biol 6, 369–375 (2010). https://doi.org/10.1038/nchembio.349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.349
This article is cited by
-
Critical contributions of pre-S1 shoulder and distal TRP box in DAG-activated TRPC6 channel by PIP2 regulation
Scientific Reports (2022)
-
Improving a genetically encoded voltage indicator by modifying the cytoplasmic charge composition
Scientific Reports (2017)
-
Allosteric substrate switching in a voltage-sensing lipid phosphatase
Nature Chemical Biology (2016)
-
A lipid two-step
Nature Chemical Biology (2016)
-
Voltage Sensing in Membranes: From Macroscopic Currents to Molecular Motions
The Journal of Membrane Biology (2015)