Abstract
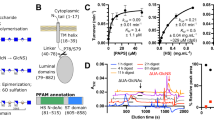
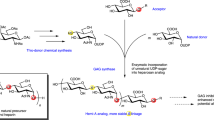
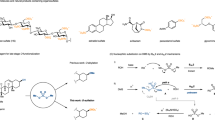
The biosynthesis of heparan sulfate (HS) involves an array of specialized sulfotransferases. Here, we present a study aimed at engineering the substrate specificity of different HS 3-O-sulfotransferase isoforms. Based on the crystal structures, we identified a pair of amino acid residues responsible for selecting the substrates. Mutations of these residues altered the substrate specificities. Our results demonstrate the feasibility of tailoring the specificity of sulfotransferases to modify HS with desired functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Liu, J. & Pedersen, L.C. Appl. Microbiol. Biotechnol. 74, 263–272 (2007).
Gama, C. et al. Nat. Chem. Biol. 2, 467–473 (2006).
Shriver, Z., Raguram, S. & Sasisekharan, R. Nat. Rev. Drug Discov. 3, 863–873 (2004).
Chen, J., Jones, C.L. & Liu, J. Chem. Biol. 14, 986–993 (2007).
Kuberan, B., Lech, M.Z., Beeler, D.L., Wu, Z.L. & Rosenberg, R.D. Nat. Biotechnol. 21, 1343–1346 (2003).
Kakuta, Y., Pedersen, L.G., Carter, C.W., Negishi, M. & Pedersen, L.C. Nat. Struct. Biol. 4, 904–908 (1997).
Kakuta, Y., Sueyoshi, T., Negishi, M. & Pedersen, L.C. J. Biol. Chem. 274, 10673–10676 (1999).
Moon, A. et al. J. Biol. Chem. 279, 45185–45193 (2004).
Mikhailov, D., Mayo, K.H., Pervin, A. & Linhardt, R.J. Biochem. J. 315, 447–454 (1996).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Merrit, E.A. & Bacon, D.J. Methods Enzymol. 277, 505–524 (1997).
DeLano, W.L. The PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, California, 2002).
Acknowledgements
The authors thank G. Cohen and R. Eisenberg (University of Pennsylvania) for providing gD-1 (306t) and anti-gD antibody. We also thank H. Kohn, L.G. Pedersen and T. Hall for reviewing the manuscript. This work is supported in part by a US National Institutes of Health grant (AI50050 to J.L.) and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (to L.C.P.). D.X. is a recipient of a predoctoral fellowship from the American Heart Association, MidAtlantic Affiliate.
Author information
Authors and Affiliations
Contributions
D.X., D.S. and J.L. prepared and characterized 3-OST mutants. A.F.M. and L.C.P. performed crystallization of 3-OST-5 and subsequent data analysis. D.X., A.F.M., L.C.P. and J.L. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1–5 and Supplementary Methods (PDF 859 kb)
Rights and permissions
About this article
Cite this article
Xu, D., Moon, A., Song, D. et al. Engineering sulfotransferases to modify heparan sulfate. Nat Chem Biol 4, 200–202 (2008). https://doi.org/10.1038/nchembio.66
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.66
This article is cited by
-
The construction of a dual-functional strain that produces both polysaccharides and sulfotransferases
Biotechnology Letters (2021)
-
Directed aryl sulfotransferase evolution toward improved sulfation stoichiometry on the example of catechols
Applied Microbiology and Biotechnology (2019)
-
Structural characterization of heparins from different commercial sources
Analytical and Bioanalytical Chemistry (2011)
-
The importance of heparan sulfate in herpesvirus infection
Virologica Sinica (2008)