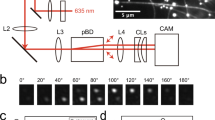
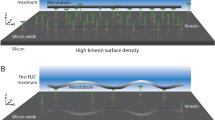
Abstract
Kinesin-1 is a twin-headed molecular motor that moves along microtubules in 8-nm steps, using a walking action in which the two heads interact alternately with the microtubule1,2,3,4. Constructs with only one head can also produce impulses of force and motion5,6,7, indicating that the walking action is an amplification strategy that leverages an underlying force-generating event. Recent work suggests that directional force is produced either by directionally biased selection of microtubule binding sites8,9 or by a conformational change subsequent to the binding event10,11,12. We report here that surface-attached rat kinesin-1 monomers drive counterclockwise rotation of sliding microtubules around their axes, and that by manipulating the assay geometry, we could reduce or block the torsional motion with negligible effects on the axial motion. We can account for this behavior on the simple assumption that kinesin heads tend to bind to the closest available tubulin heterodimer in the lattice, but only in the case where an additional biasing process is present that shifts the start position for diffusion-to-capture toward the microtubule plus end by ∼1 nm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kaseda, K., Higuchi, H. & Hirose, K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat. Cell Biol. 5, 1079–1082 (2003).
Asbury, C.L., Fehr, A.N. & Block, S.M. Kinesin moves by an asymmetric hand-over-hand mechanism. Science 302, 2130–2134 (2003).
Yildiz, A., Tomishige, M., Vale, R.D. & Selvin, P.R. Kinesin walks hand-over-hand. Science 303, 676–678 (2004).
Higuchi, H., Bronner, C.E., Park, H.W. & Endow, S.A. Rapid double 8-nm steps by a kinesin mutant. EMBO J. 23, 2993–2999 (2004).
Stewart, R.J., Thaler, J.P. & Goldstein, L.S. Direction of microtubule movement is an intrinsic property of the motor domains of kinesin heavy chain and Drosophila ncd protein. Proc. Natl. Acad. Sci. USA 90, 5209–5213 (1993).
Berliner, E., Young, E.C., Anderson, K., Mahtani, H.K. & Gelles, J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature 373, 718–721 (1995).
Young, E.C., Mahtani, H.K. & Gelles, J. One-headed kinesin derivatives move by a nonprocessive, low-duty ratio mechanism unlike that of two-headed kinesin. Biochemistry 37, 3467–3479 (1998).
Okada, Y., Higuchi, H. & Hirokawa, N. Processivity of the single-headed kinesin KIF1A through biased binding to tubulin. Nature 424, 574–577 (2003).
Kamei, T., Kakuta, S. & Higuchi, H. Biased binding of single molecules and continuous movement of multiple molecules of truncated single-headed kinesin. Biophys. J. 88, 2068–2077 (2005).
Rice, S. et al. A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784 (1999).
Kikkawa, M. et al. Switch-based mechanism of kinesin motors. Nature 411, 439–445 (2001).
Nitta, R., Kikkawa, M., Okada, Y. & Hirokawa, N. KIF1A alternately uses two loops to bind microtubules. Science 305, 678–683 (2004).
Crevel, I.M., Lockhart, A. & Cross, R.A. Weak and strong states of kinesin and ncd. J. Mol. Biol. 257, 66–76 (1996).
Okada, Y. & Hirokawa, N. Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc. Natl. Acad. Sci. USA 97, 640–645 (2000).
Yajima, J., Alonso, M.C., Cross, R.A. & Toyoshima, Y.Y. Direct long-term observation of kinesin processivity at low load. Curr. Biol. 12, 301–306 (2002).
Hancock, W.O. & Howard, J. Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. USA 96, 13147–13152 (1999).
Ray, S., Meyhofer, E., Milligan, R.A. & Howard, J. Kinesin follows the microtubule's protofilament axis. J. Cell Biol. 121, 1083–1093 (1993).
Linck, R.W. & Amos, L.A. The hands of helical lattices in flagellar doublet microtubules. J. Cell Sci. 14, 551–559 (1974).
Kadonaga, J.T., Carner, K.R., Masiarz, F.R. & Tjian, R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell 51, 1079–1090 (1987).
Hirose, K., Lockhart, A., Cross, R.A. & Amos, L.A. Nucleotide-dependent angular change in kinesin motor domain bound to tubulin. Nature 376, 277–279 (1995).
Cross, R.A. et al. The conformational cycle of kinesin. Phil. Trans. R. Soc. Lond. B 355, 459–464 (2000).
Alonso, M.C., van Damme, J., Vandekerckhove, J. & Cross, R.A. Proteolytic mapping of kinesin/ncd-microtubule interface: nucleotide-dependent conformational changes in the loops L8 and L12. EMBO J. 17, 945–951 (1998).
Naber, N. et al. EPR spectroscopy shows a microtubule-dependent conformational change in the kinesin switch 1 domain. Biophys. J. 84, 3190–3196 (2003).
Kull, F.J. & Endow, S.A. Kinesin: switch I & II and the motor mechanism. J. Cell Sci. 115, 15–23 (2002).
Vale, R.D. & Milligan, R.A. The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95 (2000).
Cross, R.A. The kinetic mechanism of kinesin. Trends Biochem. Sci. 29, 301–309 (2004).
Walker, R.A., Salmon, E.D. & Endow, S.A. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature 347, 780–782 (1990).
Acknowledgements
We thank D. Drummond, N. Carter, P. Gross and T. Oelgeschlager for advice. Supported by Marie Curie Cancer Care, the Japan Society for the Promotion of Science and the Oxford IRC in Bionanotechnology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
RK340-Sp1 binding to DNA (a) Gel mobility shift assay of RK340-Sp1 binding to an 80 bp double stranded DNA that included the Sp1 GC box recognition sequence (0, 1, 5, 10, 20, 40, 70, and 100 nM proteins in lane 0-7, respectively). (b) Apparent equilibrium binding curve obtained by calculating the fraction of DNA bound at varying RK340-Sp1 concentrations. The binding constant was determined by fitting to the equation Øb = [protein] / ([protein] + Kd) to obtain the dissociation constant (Kd). The observed dissociation constant was Kd = 21 ± 6 nM, which was close to the value as reported previously [Richard W. et al. (1992) PNAS 89 9759-63]. (PDF 588 kb)
Supplementary Video 1
Microtubule rotation driven by RK340Gel. The scale bar is 2 μm. The time code is hours : minutes : seconds : centiseconds. (MOV 4247 kb)
Supplementary Video 2
Sliding of a microtubule blocked in rotation. The arrow moving south-north marks a microtubule with a long side-arm that is unable to rotate. Sliding continues (see text) at close to wild type velocity. The arrow moving west-east tracks a microtubule with a short side-arm that is rotating normally. Time code and scale bar as in Supplementary Video 1. (MOV 2939 kb)
Supplementary Video 3
Microtubule rotation driven by RK345L-Sp1-biotinylated DNA. Time code and scale bar as in Supplementary Video 1. (MOV 4240 kb)
Rights and permissions
About this article
Cite this article
Yajima, J., Cross, R. A torque component in the kinesin-1 power stroke. Nat Chem Biol 1, 338–341 (2005). https://doi.org/10.1038/nchembio740
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio740
This article is cited by
-
Membrane-bound myosin IC drives the chiral rotation of the gliding actin filament around its longitudinal axis
Scientific Reports (2023)
-
Anchoring geometry is a significant factor in determining the direction of kinesin-14 motility on microtubules
Scientific Reports (2022)
-
Motor generated torque drives coupled yawing and orbital rotations of kinesin coated gold nanorods
Communications Biology (2022)
-
CYK4 relaxes the bias in the off-axis motion by MKLP1 kinesin-6
Communications Biology (2021)
-
Formation of helical membrane tubes around microtubules by single-headed kinesin KIF1A
Nature Communications (2015)