Read our April issue
This month, a Perspective on improving data management in scientific publications, the usual mix of Articles and News and Views, and an In Your Element on serotonin, the ‘happy hormone’

This month, a Perspective on improving data management in scientific publications, the usual mix of Articles and News and Views, and an In Your Element on serotonin, the ‘happy hormone’

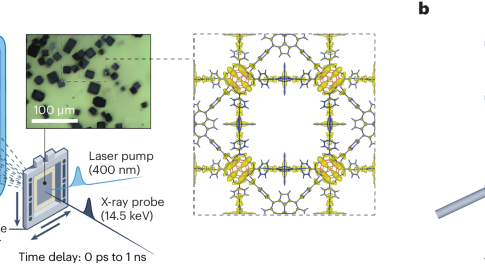
X-ray diffraction analysis typically affords the static 3D structures of given compounds or materials, but to understand chemical processes, the visualization of fast structural changes is desirable. Time-resolved femtosecond crystallography has now been used to monitor the structural dynamics of a photoactive metal–organic framework.