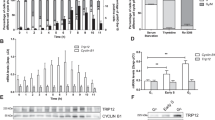
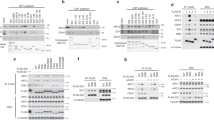
Abstract
The proliferating cell nuclear antigen (PCNA) is an essential protein for DNA replication and damage repair. How its function is controlled remains an important question. Here, we show that the chromatin-bound PCNA protein is phosphorylated on Tyr 211, which is required for maintaining its function on chromatin and is dependent on the tyrosine kinase activity of EGF receptor (EGFR) in the nucleus. Phosphorylation on Tyr 211 by EGFR stabilizes chromatin-bound PCNA protein and associated functions. Consistently, increased PCNA Tyr 211 phosphorylation coincides with pronounced cell proliferation, and is better correlated with poor survival of breast cancer patients, as well as nuclear EGFR in tumours, than is the total PCNA level. These results identify a novel nuclear mechanism linking tyrosine kinase receptor function with the regulation of the PCNA sliding clamp.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kelman, Z. & O'Donnell, M. Structural and functional similarities of prokaryotic and eukaryotic DNA polymerase sliding clamps. Nucleic Acids Res. 23, 3613–3620 (1995).
Bravo, R. & Macdonald-Bravo, H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J. Cell Biol. 105, 1549–1554 (1987).
Paunesku, T. et al. Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int. J. Radiat. Biol. 77, 1007–1021 (2001).
Haracska, L., Torres-Ramos, C.A., Johnson, R.E., Prakash, S. & Prakash, L. Opposing effects of ubiquitin conjugation and SUMO modification of PCNA on replicational bypass of DNA lesions in Saccharomyces cerevisiae. Mol. Cell. Biol. 24, 4267–4274 (2004).
Kannouche, P.L. & Lehmann, A.R. Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle 3, 1011–1013 (2004).
Jonsson, Z.O., Podust, V.N., Podust, L.M. & Hubscher, U. Tyrosine 114 is essential for the trimeric structure and the functional activities of human proliferating cell nuclear antigen. EMBO J. 14, 5745–5751 (1995).
Maga, G. & Hubscher, U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J. Cell Sci. 116, 3051–3060 (2003).
Yarden, Y. & Sliwkowski, M.X. Untangling the ErbB signalling network. Nature Rev. Mol. Cell Biol. 2, 127–137 (2001).
Lin, S.Y. et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nature Cell Biol. 3, 802–808 (2001).
Lo, H.-W. et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 7, 575–589 (2005).
Hanada, N. et al. Co-regulation of b-Myb expression by E2F1 and EGF receptor. Mol. Carcinog. 45, 10–17 (2006).
Assy, N. & Minuk, G.Y. Liver regeneration: methods for monitoring and their applications. J. Hepatol. 26, 945–952 (1997).
Marti, U. & Hug, M. Acinar and cellular distribution and mRNA expression of the epidermal growth factor receptor are changed during liver regeneration. J. Hepatol. 23, 318–327 (1995).
Psyrri, A. et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin. Cancer Res. 11, 5856–5862 (2005).
Marti, U. et al. Nuclear localization of epidermal growth factor and epidermal growth factor receptors in human thyroid tissues. Thyroid 11, 137–145 (2001).
Tao, Y. et al. Nuclear accumulation of epidermal growth factor receptor and acceleration of G1/S stage by Epstein–Barr-encoded oncoprotein latent membrane protein 1. Exp. Cell Res. 303, 240–251 (2005).
Lo, H.-W. et al. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 65, 338–348 (2005).
Jeziorski, A., Blonski, J.Z. & Niewiadomska, H. The expression of products of oncogens c-erbB2 and EGFR and proliferating antigens Ki67 and PCNA in primary invasive ductal cancer of female breast. J. Exp. Clin. Cancer Res. 19, 61–67 (2000).
Shrestha, P. et al. Proliferating cell nuclear antigen in breast lesions: correlation of c-erbB-2 oncoprotein and EGF receptor and its clinicopathological significance in breast cancer. Virchows Arch. A Pathol. Anat. Histopathol. 421, 193–202 (1992).
Frassoldati, A. et al. Changes of biological features in breast cancer cells determined by primary chemotherapy. Breast Cancer Res. Treat. 44, 185–192 (1997).
Hoege, C., Pfander, B., Moldovan, G.-L., Pyrowolakis, G. & Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 (2002).
Stelter, P. & Ulrich, H.D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425, 188–191 (2003).
Ulrich, H.D. How to activate a damage-tolerant polymerase: consequences of PCNA modifications by ubiquitin and SUMO. Cell Cycle 3, 15–18 (2004).
Kiyokawa, N., Lee, E.K., Karunagaran, D., Lin, S.-Y. & Hung, M.-C. Mitosis-specific negative regulation of epidermal growth factor receptor, triggered by a decrease in ligand binding and dimerization, can be overcome by overexpression of receptor. J. Biol. Chem. 272, 18656–18665 (1997).
Ammosova, T. et al. Nuclear targeting of protein phosphatase-1 by HIV-1 Tat protein. J. Biol. Chem. 280, 36364–36371 (2005).
Hasan, S., Hassa, P.O., Imhof, R. & Hottiger, M.O. Transcription coactivator p300 binds PCNA and may have a role in DNA repair synthesis. Nature 410, 387–391 (2001).
Umar, A. et al. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell 87, 65–73 (1996).
Kato, T. et al. New prognostic factors associated with long-term survival in node-negative breast cancer patients. Breast Cancer 6, 370–377 (1999).
Kato, T., Kameoka, S., Kimura, T., Nishikawa, T. & Kobayashi, M. The combination of angiogenesis and blood vessel invasion as a prognostic indicator in primary breast cancer. Br. J. Cancer 88, 1900–1908 (2003).
Lee, J.S. et al. Correlation between angiogenesis, apoptosis and cell proliferation in invasive ductal carcinoma of the breast and their relation to tumor behavior. Anal. Quant. Cytol. Histol. 23, 161–168 (2001).
Bukholm, I.R.K., Bukholm, G., Holm, R. & Nesland, J.M. Association between histology grade, expression of HsMCM2, and cyclin A in human invasive breast carcinomas. J. Clin. Pathol. 56, 368–373 (2003).
Grossi, F. et al. Prognostic significance of K-ras, p53, bcl-2, PCNA, CD34 in radically resected non-small cell lung cancers. Eur. J. Cancer 39, 1242–1250 (2003).
Heimann, R., Ferguson, D., Recant, W.M. & Hellman, S. Breast cancer metastatic phenotype as predicted by histologic tumor markers. Cancer J. Sci. Am. 3, 224–249 (1997).
Arteaga, C.L. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin. Oncol. 29, 3–9 (2002).
Baselga, J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist 7, 2–8 (2002).
Wang, S.-C. et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell 6, 251–261 (2004).
Xie, Y.M. & Hung, M.C. Nuclear localization of p185neu tyrosine kinase and its association with transcriptional transactivation. Biochem. Biophys. Res. Commun. 203, 1589–1598 (1994).
Offterdinger, M., Schofer, C., Weipoltshammer, K. & Grunt, T.W. c-erbB-3: a nuclear protein in mammary epithelial cells. J. Cell Biol. 157, 929–940 (2002).
Ni, C.-Y., Murphy, M.P., Golde, T.E. & Carpenter, G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294, 2179–2181 (2001).
Lo, H.W. & Hung, M.C. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 94, 184–188 (2006).
Massie, C. & Mills, I.G. The developing role of receptors and adaptors. Nature Rev. Cancer 6, 403–409 (2006).
Carpenter, G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr. Opin. Cell Biol. 15, 143–148 (2003).
Wells, A. & Marti, U. Signalling shortcut: cell-surface receptors in the nucleus? Nature Rev. Mol. Cell Biol. 3, 697–702 (2002).
Lo, H.W. et al. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin β1 and CRM1. J. Cell Biochem. 98, 1570–1583 (2006).
Giri, D.K. et al. Endosomal transport of ErbB-2: a mechanism for nuclear entry of cell surface receptor. Mol. Cell. Biol. 25, 11005–11018 (2005).
Williams, C.C. et al. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J. Cell Biol. 167, 469–478 (2004).
Huang, T.T. et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nature Cell Biol. 8, 341–347 (2006).
Asada, M. et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18, 1223–1234 (1999).
Acknowledgements
The anti-phospho-Tyr 211 antibody was generated by Bethyl Laboratories, Inc. as a collaboration. We also thank S. Dent, M. Van Dyke and D. Yu for helpful discussions and comments. This study was supported, in part, by the NIH RO1 109311 and NIH PO1 099031 grants, the National Breast Cancer Foundation, Inc. (to M.-C. H.), and the Cancer Center support grant CA16672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.W.M is currently an employee of Bethyl Laboratories, Inc. (Montgomery, TX), which has a commerical interest in the antibodies against PCNA and phospho-PCNA. E.W.M. collaborated with the Hung group to develop these antibodies.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 655 kb)
Rights and permissions
About this article
Cite this article
Wang, SC., Nakajima, Y., Yu, YL. et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol 8, 1359–1368 (2006). https://doi.org/10.1038/ncb1501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1501
This article is cited by
-
Domain-specific p53 mutants activate EGFR by distinct mechanisms exposing tissue-independent therapeutic vulnerabilities
Nature Communications (2023)
-
RNA-binding protein p54nrb/NONO potentiates nuclear EGFR-mediated tumorigenesis of triple-negative breast cancer
Cell Death & Disease (2022)
-
EGFR signaling promotes nuclear translocation of plasma membrane protein TSPAN8 to enhance tumor progression via STAT3-mediated transcription
Cell Research (2022)
-
Genomic instability as a major mechanism for acquired resistance to EGFR tyrosine kinase inhibitors in cancer
Protein & Cell (2022)
-
Downregulation of the FBXO43 gene inhibits tumor growth in human breast cancer by limiting its interaction with PCNA
Journal of Translational Medicine (2021)