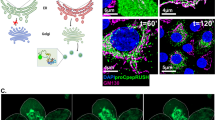
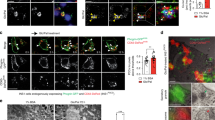
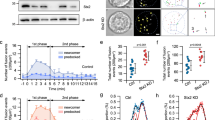
Abstract
Pancreatic β-cells store insulin in secretory granules that undergo exocytosis upon glucose stimulation. Sustained stimulation depletes β-cells of their granule pool, which must be quickly restored. However, the factors promoting rapid granule biogenesis are unknown. Here we show that β-cell stimulation induces the nucleocytoplasmic translocation of polypyrimidine tract-binding protein (PTB). Activated cytosolic PTB binds and stabilizes mRNAs encoding proteins of secretory granules, thus increasing their translation, whereas knockdown of PTB expression by RNA interference (RNAi) results in the depletion of secretory granules. These findings may provide insight for the understanding and treatment of diabetes, in which insulin secretion is typically impaired.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Borgonovo, B. et al. Neurosecretion competence, an independently regulated trait of the neurosecretory cell phenotype. J. Biol. Chem. 273, 34683–34686 (1998).
Guest, P.C., Rhodes, C.J. & Hutton, J.C. Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem. J. 257, 431–437 (1989).
Alarcon, C., Lincoln, B. & Rhodes, C.J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J. Biol. Chem. 268, 4276–4280 (1993).
Martin, S.K., Carroll, R., Benig, M. & Steiner, D.F. Regulation by glucose of the biosynthesis of PC2, PC3 and proinsulin in (ob/ob) mouse islets of Langerhans. FEBS Lett. 356, 279–282 (1994).
Giddings, S.J., Chirgwin, J. & Permutt, M.A. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes 31, 624–629 (1982).
Permutt, M.A. & Kipnis, D.M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J. Biol. Chem. 247, 1194–1199 (1972).
Itoh, N. & Okamoto, H. Translational control of proinsulin synthesis by glucose. Nature 283, 100–102 (1980).
Welsh, M., Scherberg, N., Gilmore, R. & Steiner, D.F. Translational control of insulin biosynthesis. Evidence for regulation of elongation, initiation and signal-recognition-particle-mediated translational arrest by glucose. Biochem J. 235, 459–467 (1986).
Welsh, M., Nielsen, D.A., MacKrell, A.J. & Steiner, D.F. Control of insulin gene expression in pancreatic β-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J. Biol. Chem. 260, 13590–13594 (1985).
Tillmar, L., Carlsson, C. & Welsh, N. Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3′-untranslated region pyrimidine-rich sequence. J. Biol. Chem. 277, 1099–1106 (2002).
Tillmar, L. & Welsh, N. Hypoxia may increase rat insulin mRNA levels by promoting binding of the polypyrimidine tract-binding protein (PTB) to the pyrimidine-rich insulin mRNA 3′-untranslated region. Mol. Med. 8, 263–272 (2002).
Garcia-Blanco, M.A., Jamison, S.F. & Sharp, P.A. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 3, 1874–1886 (1989).
Valcarcel, J. & Gebauer, F. Post-transcriptional regulation: the dawn of PTB. Curr. Biol. 7, R705–R708 (1997).
Hellen, C.U. et al. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl Acad. Sci. USA 90, 7642–7646 (1993).
Cote, C.A. et al. A Xenopus protein related to hnRNP I has a role in cytoplasmic RNA localization. Mol. Cell 4, 431–437 (1999).
Moreira, A. et al. The upstream sequence element of the C2 complement poly(A) signal activates mRNA 3′ end formation by two distinct mechanisms. Genes Dev. 12, 2522–2534 (1998).
Hamilton, B.J., Genin, A., Cron, R.Q. & Rigby, W.F.C. Delineation of a novel pathway that regulates CD154 (CD40 ligand) expression. Mol. Cell. Biol. 23, 510–525 (2003).
Gil, A., Sharp, P.A., Jamison, S.F. & Garcia-Blanco, M.A. Characterization of cDNAs encoding the polypyrimidine tract-binding protein. Genes Dev. 5, 1224–1236 (1991).
Ghetti, A., Pinol-Roma, S., Michael, W.M., Morandi, C. & Dreyfuss, G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 20, 3671–3678 (1992).
Solimena, M. et al. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMB0 J. 15, 2102–2114 (1996).
Ort, T. et al. Dephosphorylation of β2-syntrophin and Ca2+/mu-calpain-mediated cleavage of ICA512 upon stimulation of insulin secretion. EMBO J. 20, 4013–4023 (2001).
Lee, M.S., Dirkx, R.J., Solimena, M. & Dannies, P.S. Stabilization of the receptor protein tyrosine phosphatase-like protein ICA512 in GH4C1 cells upon treatment with estradiol, insulin, and epidermal growth factor. Endocrinology 139, 2727–2733 (1998).
Gosert, R., Chang, K.H., Rijnbrand, R., Yi, M., Sangar, D.V. & Lemon, S.M. Transient expression of cellular polypyrimidine-tract binding protein stimulates cap-independent translation directed by both picornaviral and flaviviral internal ribosome entry sites in vivo. Mol. Cell Biol. 20, 1583–1595 (2000).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Bothwell, A.L. et al. Murine polypyrimidine tract binding protein. Purification, cloning, and mapping of the RNA binding domain. J. Biol. Chem. 266, 24657–24663 (1991).
Schatz, H., Nierle, C. & Pfeiffer, E.F. (Pro-)insulin biosynthesis and release of newly synthesized (pro-)insulin from isolated islets of rat pancreas in the presence of amino acids and sulphonylureas. Eur. J. Clin. Invest. 5, 477–485 (1975).
Nielsen, D.A., Welsh, M., Casadaban, M.J. & Steiner, D.F. Control of insulin gene expression in pancreatic β-cells and in an insulin-producing cell line, RIN-5F cells. I. Effects of glucose and cyclic AMP on the transcription of insulin mRNA. J. Biol. Chem. 260, 13585–13589 (1985).
Drucker, D.J., Philippe, J., Mojsov, S., Chick, W.L. & Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl Acad. Sci. USA 84, 3434–3438 (1987).
Xie, J., Lee, J.A., Kress, T.L., Mowry, K.L. & Black, D.L. Protein kinase A phosphorylation modulates transport of the polypyrimidine tract-binding protein. Proc. Natl Acad. Sci. USA 100, 8776–8781 (2003).
Xu, G. et al. Insulin mediates glucose-stimulated phosphorylation of PHAS-I by pancreatic beta cells. An insulin-receptor mechanism for autoregulation of protein synthesis by translation. J. Biol. Chem. 273, 4485–4491 (1998).
Kim, T., Tao-Cheng, J.-H., Eiden, L.E. & Loh, Y.P. Chromogranin A, an on/off switch controlling dense-core secretory granule biogenesis. Cell 106, 499–509 (2001).
Day, R. & Gorr, S.U. Secretory granule biogenesis and chromogranin A: master gene, on/off switch or assembly factor? Trends Endocrinol. Metab. 14, 10–13 (2003).
Malosio, M.L., Giordano, T., Laslop, A. & Meldolesi, J.J. Dense-core granules: a specific hallmark of the neuronal/neurosecretory phenotype. J. Cell Sci. 117, 743–749 (2004).
Gold, G., Gishizky, M.L. & Grodsky, G.M. Evidence that glucose 'marks' β cells resulting in preferential release of newly synthesized insulin. Science 218, 56–58 (1982).
Halban, P.A. Differential rates of release of newly synthesized and of stored insulin from pancreatic islets. Endocrinology 110, 1183–1188 (1982).
Gotoh, M., Maki, T., Kiyoizumi, T., Satomi, S. & Monaco, A.P. An improved method for isolation of mouse pancreatic islets. Transplantation 40, 437–438 (1985).
Asfari, M. et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178 (1992).
Velasco, A., Hendricks, L., Moremen, K.W., Tulsiani, D.R., Touster, O. & Farquhar, M.G. Cell type-dependent variations in the subcellular distribution of α-mannosidase I and II. J. Cell Biol. 122, 39–51 (1993).
Saraste, J. & Svensson, K. Distribution of the intermediate elements operating in ER to Golgi transport. J. Cell Sci. 100, 415–430 (1991).
Wang, Z., Day, N., Trifillis, P. & Kiledjian, M. An mRNA stability complex functions with poly(A)-binding protein to stabilize mRNA in vitro. Mol. Cell. Biol. 19, 4552–4560 (1999).
Steinbrenner, H., Nguyen, T.B., Wohlrab, U., Scherbaum, W.A. & Seissler, J. Effect of proinflammatory cytokines on gene expression of the diabetes-associated autoantigen IA-2 in INS-1 cells. Endocrinology 143, 3839–3845 (2002).
Acknowledgements
We thank P. De Camilli, C. Walch-Solimena and E. Ullu for critical reading of the manuscript, R. Jahn, W. Huttner, K. Moremen, A. Helenius and J. Saraste for antibodies, M. Kolpe and R. Meisterfeld for help with islet isolation and insulin radioimmunoassay, F. Theissig for preparing islet sections, F. Bucholz for advice on RNAi, K. Scheckel for providing Jurkat cells, C. Echeverri and L. Buffa for siRNA oligonucleotides, K. Pfriem for excellent secretarial assistance and M. Füssel for support. We are also very grateful to the reviewers, whose criticisms greatly improved this article. This work was supported by grants from the A. von Humboldt Foundation and the Bundesministerium für Bildung und Forschung (BMBF) to M.S. and by a Telethon Post-Doctoral Fellowship to B.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have a patent application pending.
Supplementary information
Rights and permissions
About this article
Cite this article
Knoch, KP., Bergert, H., Borgonovo, B. et al. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat Cell Biol 6, 207–214 (2004). https://doi.org/10.1038/ncb1099
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1099
This article is cited by
-
RBP–RNA interactions in the control of autoimmunity and autoinflammation
Cell Research (2023)
-
Neonatal cortical astrocytes possess intrinsic potential in neuronal conversion in defined media
Acta Pharmacologica Sinica (2021)
-
The making of insulin in health and disease
Diabetologia (2020)
-
Circular RNA FOXP1 promotes tumor progression and Warburg effect in gallbladder cancer by regulating PKLR expression
Molecular Cancer (2019)
-
Lysosomal degradation of newly formed insulin granules contributes to β cell failure in diabetes
Nature Communications (2019)