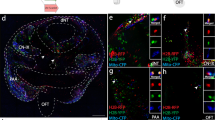
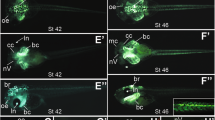
Abstract
Previous analyses of labelled clones of cells within the developing nervous system of the mouse have indicated that descendants are initially dispersed rostrocaudally followed by more local proliferation, which is consistent with the progressing node's contributing descendants from a resident population of progenitor cells as it advances caudally. Here we electroporated an expression vector encoding green fluorescent protein into the chicken embryo near Hensen's node to test and confirm the pattern inferred in the mouse. This provides a model in which a proliferative stem zone is maintained in the node by a localized signal; those cells that are displaced out of the stem zone go on to contribute to the growing axis. To test whether fibroblast growth factor (FGF) signalling could be involved in the maintenance of the stem zone, we co-electroporated a dominant-negative FGF receptor with a lineage marker, and found that it markedly alters the elongation of the spinal cord primordium. The results indicate that FGF receptor signalling promotes the continuous development of the posterior nervous system by maintaining presumptive neural progenitors in the region near Hensen's node. This offers a potential explanation for the mixed findings on FGF in the growth and patterning of the embryonic axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lumsden, A. & Krumlauf, R. Patterning the vertebrate neuraxis. Science 274, 1109–1114 (1996).
Harland, R. & Gerhart, J. Formation and function of Spemann's organizer. Annu. Rev. Cell. Dev. Biol. 13, 611–667 (1997).
Smith, J. L. & Schoenwolf, G. C. Getting organized: new insights into the organizer of higher vertebrates. Curr. Top. Dev. Biol. 40, 79–110 (1998).
Woo, K. & Fraser, S. E. Specification of the zebrafish nervous system by nonaxial signals. Science 277, 254–257 (1997).
Rowan, A. M., Stern, C. D. & Storey, K. G. Axial mesendoderm refines rostrocaudal pattern in the chick nervous system. Development 126, 2921–2934 (1999).
Bouwmeester, T. & Leyns, L. Vertebrate head induction by anterior primitive endoderm. BioEssays 19, 855–863 (1997).
Beddington, R. S. & Robertson, E. J. Axis development and early asymmetry in mammals. Cell 96, 195–209 (1999).
Woo, K. & Fraser, S. E. Specification of the hindbrain fate in the zebrafish. Dev. Biol. 197, 283–296 (1998).
Darnell, D. K., Stark, M. R. & Schoenwolf, G. C. Timing and cell interactions underlying neural induction in the chick embryo. Development 126, 2505–2514 (1999).
Nieuwkoop, P. D. Activation and organization of the central nervous system in amphibians. Part III. Synthesis of a new working hypothesis. J. Exp. Zool. 120, 83–108 (1952).
Doniach, T. Basic FGF as an inducer of anteroposterior neural pattern. Cell 83, 1067–1070 (1995).
Lamb, T. M. & Harland, R. M. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior–posterior neural pattern. Development 121, 3627–3636 (1995).
Cox, W. G. & Hemmati-Brivanlou, A. Caudalization of neural fate by tissue recombination and bFGF. Development 121, 4349–4358 (1995).
Kengaku, M. & Okamoto, H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development 121, 3121–3130 (1995).
Kroll, K. L. & Amaya, E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173–3183 (1996).
Keller, R. & Danilchik, M. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development 103, 193–209 (1988).
Mathis, L. & Nicolas, J. F. Different clonal dispersion in the rostral and caudal mouse central nervous system. Development 127, 1277–1290 (2000).
Schoenwolf, G. C. & Alvarez, I. S. Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development 106, 427–439 (1989).
Alvarez, I. S. & Schoenwolf, G. C. Patterns of neurepithelial cell rearrangement during avian neurulation are determined prior to notochordal inductive interactions. Dev. Biol. 143, 78–92 (1991).
Hatada, Y. & Stern, C. D. A fate map of the epiblast of the early chick embryo. Development 120, 2879–2889 (1994).
Catala, M., Teillet, M. A., De Robertis, E. M. & Le Douarin, M. L. A spinal cord fate map in the avian embryo: while regressing, Hensen's node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development 122, 2599–2610 (1996).
Henrique, D. et al. cash4, a novel achaete-scute homolog induced by Hensen's node during generation of the posterior nervous system. Genes Dev. 11, 603–615 (1997).
Kimmel, C. B., Warga, R. M. & Kane, D. A. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development 120, 265–276 (1994).
Woo, K. & Fraser, S. E. Order and coherence in the fate map of the zebrafish nervous system. Development 121, 2595–2609 (1995).
Eagleson, G. W. & Harris, W. A. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J. Neurobiol. 21, 427–440 (1990).
Keller, R., Shih, J. & Sater, A. The cellular basis of the convergence and extension of the Xenopus neural plate. Dev. Dyn. 193, 199–217 (1992).
Itasaki, N., Bel, V. S. & Krumlauf, R. 'Shocking' developments in chick embryology: electroporation and in ovo gene expression. Nature Cell Biol. 1, E203–E207 (1999).
Schoenwolf, G. C. Observations on closure of the neuropores in the chick embryo. Am. J. Anat. 4, 445–465 (1979).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Ueno, H., Gunn, M., Dell, K., Tseng, A., Jr, Williams, L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J. Biol. Chem. 267, 1470–1476 (1992).
Amaya, E., Musci, T. J. & Kirschner, M. W. Expression of a dominant-negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66, 257–270 (1991).
Zou, H. & Niswander, L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 272, 738–741 (1996).
Selleck, M. A. & Stern, C. D. in Formation and Differentiation of Early Embryonic Mesoderm (ed. R. Bellairs) 23–31 (Plenum, New York, 1992).
Beddington, R. S. Induction of a second neural axis by the mouse node. Development 120, 613–620 (1994).
Nicolas, J. F., Mathis, L. & Bonnerot, C. Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development 122, 2933–2946 (1996).
Psychoyos, D. & Stern, C. D. Fates and migratory routes of primitive streak cells in the chick embryo. Development 122, 1523–1534 (1996).
Davidson, B. P., Kinder, S. J., Steiner, K., Schoenwolf, G. C. & Tam, P. P. Impact of node ablation on the morphogenesis of the body axis and the lateral asymmetry of the mouse embryo during early organogenesis. Dev. Biol. 211(1), 11–26 (1999).
Brown, J. M. & Storey, K. G. A region of the vertebrate neural plate in which neighbouring cells can adopt neural or epidermal fates. Curr. Biol. 10, 869–872 (2000).
Yamaguchi, T. P., Conlon, R. A. & Rossant, J. Expression of the fibroblast growth factor receptor FGFR-1/flg during gastrulation and segmentation in the mouse embryo. Dev. Biol. 152, 75–88 (1992).
Slack, J. M., Isaacs, H. V., Song, J., Durbin, L. & Pownall, M. E. The role of fibroblast growth factors in early Xenopus development. Biochem. Soc. Symp. 62, 1–12 (1996).
Storey, K. G. et al. Early posterior neural tissue is induced by FGF in the chick embryo. Development 125, 473–484 (1998).
Pownall, M. E., Tucker, A. S., Slack, J. M. & Isaacs, H. V. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 122, 3881–3892 (1996).
Holowacz, T. & Sokol, S. FGF is required for posterior neural patterning but not for neural induction. Dev. Biol. 205, 296–308 (1999).
Godsave, S. F. & Durston, A. J. Neural induction and patterning in embryos deficient in FGF signaling. Int. J. Dev. Biol. 41, 57–65 (1997).
Koshida, S., Shinya, M., Mizuno, T., Kuroiwa, A. & Takeda, H. Initial anteroposterior pattern of the zebrafish central nervous system is determined by differential competence of the epiblast. Development 125, 1957–1966 (1998).
Keller, R., Shih, J., Sater, A. K. & Moreno, C. Planar induction of convergence and extension of the neural plate by the organizer of Xenopus. Dev. Dyn. 193, 218–234 (1992).
Yamada, T. Caudalization by the amphibian organizer: brachyury, convergent extension and retinoic acid. Development 120, 3051–3062 (1994).
Yates, J. L., Warren, N. & Sugden, B. Stable replication of plasmids derived from Epstein–Barr virus in various mammalian cells. Nature 313, 812–815 (1985).
Okada, A., Lansford, R., Weimann, J. M., Fraser, S. E. & McConnell, S. K. Imaging cells in the developing nervous system with retrovirus expressing modified green fluorescent protein. Exp. Neurol. 156, 394–406 (1999).
Mombaerts, P. et al. Visualizing an olfactory sensory map. Cell 87, 675–686 (1996).
Mathis, L., Sieur, J., Voiculescu, O., Charnay, P. & Nicolas, J. F. Successive patterns of clonal cell dispersion in relation to neuromeric subdivision in the mouse neuroepithelium. Development 126, 4095–4106 (1999).
Kulesa, P. M. & Fraser, S. E. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development 127, 1161–1172 (2000).
Kulesa, P. M. & Fraser, S. E. Neural crest cell dynamics revealed by time-lapse video microscopy. Dev. Biol. 15, 327–344 (1998).
Acknowledgements
We thank R. Lansford, E. Dorman, E. Amaya and L. Niswander for reagents, M. Bronner-Fraser, M. Garcia-Castro, A. Knecht and H. McBride for comments on the manuscript, members of the Fraser laboratory for advice, and J. Horn for his help with electroporation. L.M. is the recipient of an EMBO long-term fellowship; P.M.K. is a participant in the California Institute of Technology Initiative in Computational Molecular Biology, which is funded by a Burroughs Wellcome Fund Interfaces award.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
41556_2001_BFncb0601_559_MOESM2_ESM.qt
Movie 1 Elongation motion in Hensen's node region fated to the neurectoderm. Time-lapse analysis of the GFP population comprising the posterior neural plate and the node region. Frames are 5 min apart. Cell behaviours of this movie are summarized in Fig. 3a. (QT 1265 kb)
41556_2001_BFncb0601_559_MOESM3_ESM.qt
Movie 2 Coherent behaviour of neural progenitors. Two adjacent GFPexpressing cells retain their relative locations (coherence) during Hensen's node progression. (QT 2690 kb)
41556_2001_BFncb0601_559_MOESM4_ESM.qt
Movie 3 Elongation motion in Hensen's node region fated to the mesoderm. Clusters of axial (mesoderm) progenitors labelled by DiI become progressively displaced from each other while individual cells migrate away into the mesoderm. (QT 346 kb)
Rights and permissions
About this article
Cite this article
Mathis, L., Kulesa, P. & Fraser, S. FGF receptor signalling is required to maintain neural progenitors during Hensen's node progression. Nat Cell Biol 3, 559–566 (2001). https://doi.org/10.1038/35078535
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35078535
This article is cited by
-
Molecular control of macroscopic forces drives formation of the vertebrate hindgut
Nature (2019)
-
Dynamics and mechanisms of posterior axis elongation in the vertebrate embryo
Cellular and Molecular Life Sciences (2019)
-
Modeling ALS with motor neurons derived from human induced pluripotent stem cells
Nature Neuroscience (2016)
-
Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells
Nature (2011)
-
NOGO-A induction and localization during chick brain development indicate a role disparate from neurite outgrowth inhibition
BMC Developmental Biology (2007)