Abstract
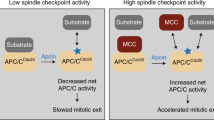
Progress through mitosis requires that the right protein be degraded at the right time. One ubiquitin ligase, the anaphase-promoting complex or cyclosome (APC/C) targets most of the crucial mitotic regulators by changing its substrate specificity throughout mitosis. The spindle assembly checkpoint (SAC) acts on the APC/C co-activator, Cdc20 (cell division cycle 20), to block the degradation of metaphase substrates (for example, cyclin B1 and securin), but not others (for example, cyclin A). How this is achieved is unclear. Here we show that Cdc20 binds to different sites on the APC/C depending on the SAC. Cdc20 requires APC3 and APC8 to bind and activate the APC/C when the SAC is satisfied, but requires only APC8 to bind the APC/C when the SAC is active. Moreover, APC10 is crucial for the destruction of cyclin B1 and securin, but not cyclin A. We conclude that the SAC causes Cdc20 to bind to different sites on the APC/C and this alters APC/C substrate specificity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
31 March 2011
In the version of this article initially published online and in print, there were some data errors in table 1. These errors have been corrected in the HTML and PDF versions of the article.
References
Pines, J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 (2006).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644–656 (2006).
Thornton, B. R. et al. An architectural map of the anaphase-promoting complex. Genes Dev. 20, 449–460 (2006).
Gmachl, M., Gieffers, C., Podtelejnikov, A. V., Mann, M. & Peters, J. M. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl Acad. Sci. USA 97, 8973–8978 (2000).
Tang, Z. et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12, 3839–3851 (2001).
Schwab, M., Neutzner, M., Mocker, D. & Seufert, W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20, 5165–5175 (2001).
Sorensen, C. S. et al. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex–Cdh1 and cyclin A–Cdk2 during cell cycle progression. Mol. Cell. Biol. 21, 3692–3703 (2001).
Burton, J. L. & Solomon, M. J. Hsl1p, a swe1p inhibitor, is degraded via the anaphase-promoting complex. Mol. Cell. Biol. 20, 4614–4625 (2000).
Kraft, C., Vodermaier, H. C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell 18, 543–553 (2005).
Hilioti, Z., Chung, Y., Mochizuki, Y., Hardy, C. F. & Cohen-Fix, O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 11, 1347–1352 (2001).
Pfleger, C. M., Lee, E. & Kirschner, M. W. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15, 2396–2407 (2001).
Mallory, M. J., Cooper, K. F. & Strich, R. Meiosis-specific destruction of the Ume6p repressor by the Cdc20-directed APC/C. Mol. Cell 27, 951–961 (2007).
Kimata, Y. et al. A mutual inhibition between APC/C and its substrate Mes1 required for meiotic progression in fission yeast. Dev. Cell 14, 446–454 (2008).
Eytan, E., Moshe, Y., Braunstein, I. & Hershko, A. Roles of the anaphase-promoting complex/cyclosome and of its activator Cdc20 in functional substrate binding. Proc. Natl Acad. Sci. USA 103, 2081–2086 (2006).
Passmore, L. A. & Barford, D. Coactivator functions in a stoichiometric complex with anaphase-promoting complex/cyclosome to mediate substrate recognition. EMBO Rep. 6, 873–878 (2005).
Sullivan, M. & Morgan, D. O. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 8, 894–903 (2007).
Pfleger, C. M. & Kirschner, M. W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–665 (2000).
Blanco, M. A., Sanchez-Diaz, A., de Prada, J. M. & Moreno, S. APCste9−srw1 promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J. 19, 3945–3955 (2000).
Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
Sigrist, S. J. & Lehner, C. F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90, 671–681 (1997).
Sudo, T. et al. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 20, 6499–6508 (2001).
Li, M. et al. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat. Cell Biol. 10, 1083–1089 (2008).
Garcia-Higuera, I. et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat. Cell Biol. 10, 802–811 (2008).
Floyd, S., Pines, J. & Lindon, C. APC/CCdh1 targets aurora kinase to control reorganization of the mitotic spindle at anaphase. Curr. Biol. 18, 1649–1658 (2008).
Rape, M., Reddy, S. K. & Kirschner, M. W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 (2006).
Summers, M. K., Pan, B., Mukhyala, K. & Jackson, P. K. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol. Cell 31, 544–556 (2008).
Rape, M. & Kirschner, M. W. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 432, 588–595 (2004).
Walker, A., Acquaviva, C., Matsusaka, T., Koop, L. & Pines, J. UbcH10 has a rate-limiting role in G1 phase but might not act in the spindle checkpoint or as part of an autonomous oscillator. J. Cell Sci. 121, 2319–2326 (2008).
Clute, P. & Pines, J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1, 82–87 (1999).
den Elzen, N. & Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121–136 (2001).
Geley, S. et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153, 137–148 (2001).
Hames, R. S., Wattam, S. L., Yamano, H., Bacchieri, R. & Fry, A. M. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 20, 7117–7127 (2001).
Di Fiore, B. & Pines, J. How cyclin A destruction escapes the spindle assembly checkpoint. J. Cell Biol. 190, 501–509 (2010).
Wolthuis, R. et al. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell 30, 290–302 (2008).
Hayes, M. J. et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 8, 607–614 (2006).
Vodermaier, H. C., Gieffers, C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13, 1459–1468 (2003).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Ohta, T., Michel, J. J., Schottelius, A. J. & Xiong, Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol. Cell 3, 535–541 (1999).
Zachariae, W. & Nasmyth, K. TPR proteins required for anaphase progression mediate ubiquitination of mitotic B-type cyclins in yeast. Mol. Biol. Cell 7, 791–801 (1996).
Deak, P., Donaldson, M. & Glover, D. M. Mutations in makos, aDrosophila gene encoding the Cdc27 subunit of the anaphase promotingcomplex, enhance centrosomal defects in polo and are suppressed by mutations in twins-aar, which encodes a regulatory subunit of PP2A. J. Cell Sci. 116, 4147–4158 (2003).
Carroll, C. W., Enquist-Newman, M. & Morgan, D. O. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 15, 11–18 (2005).
Matyskiela, M. E. & Morgan, D. O. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol. Cell 34, 68–80 (2009).
Passmore, L. A. et al. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 22, 786–796 (2003).
Kimata, Y., Baxter, J. E., Fry, A. M. & Yamano, H. A role for the Fizzy-Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol. Cell 32, 576–583 (2008).
Herzog, F. et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science 323, 1477–1481 (2009).
Zhang, Z. et al. Molecular structure of the N-terminal domain of the APC/C subunit Cdc27 reveals a homo-dimeric tetratricopeptide repeat architecture. J. Mol. Biol. 397, 1316–1328 (2010).
Dube, P. et al. Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol. Cell 20, 867–879 (2005).
Yu, H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol. Cell 27, 3–16 (2007).
Nilsson, J., Yekezare, M., Minshull, J. & Pines, J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10, 1411–1420 (2008).
Steen, J. A. et al. Different phosphorylation states of the anaphase promoting complex in response to antimitotic drugs: a quantitative proteomic analysis. Proc. Natl Acad. Sci. USA 105, 6069–6074 (2008).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 14, 111–122 (2008).
Kulukian, A., Han, J. S. & Cleveland, D. W. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell 16, 105–117 (2009).
Tang, Z., Bharadwaj, R., Li, B. & Yu, H. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1, 227–237 (2001).
van Zon, W. et al. The APC/C recruits cyclin B1–Cdk1–Cks in prometaphase before D box recognition to control mitotic exit. J. Cell Biol. 190, 587–602 (2010).
Burton, J. L. & Solomon, M. J. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 21, 655–667 (2007).
Pines, J. & Hunter, T. Isolation of a human cyclin cDNA: Evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58, 833–846 (1989).
Acknowledgements
We thank J-M. Peters for the anti-phospho APC1 antibody and S. Taylor for the HeLa-FRT cell line. We are grateful to D. Barford for discussing results before publication and all the members of our laboratory for comments and criticisms. D.I. was supported by a fellowship from the Japanese Society for the Promotion of Science and by the Association for International Cancer Research (AICR). The work was supported by core funding to the Gurdon Institute from the Wellcome Trust and Cancer Research UK, by a project grant from the AICR and a programme grant from Cancer Research UK to J.P.
Author information
Authors and Affiliations
Contributions
D.I. and J.P. designed and interpreted the experiments; D.I. carried out and analysed all the experiments; D.I. and J.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1363 kb)
Rights and permissions
About this article
Cite this article
Izawa, D., Pines, J. How APC/C–Cdc20 changes its substrate specificity in mitosis. Nat Cell Biol 13, 223–233 (2011). https://doi.org/10.1038/ncb2165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2165
This article is cited by
-
CDK9-55 guides the anaphase-promoting complex/cyclosome (APC/C) in choosing the DNA repair pathway choice
Oncogene (2024)
-
Time varying causal network reconstruction of a mouse cell cycle
BMC Bioinformatics (2019)
-
Chromosome integrity checkpoints in stem and progenitor cells: transitions upon differentiation, pathogenesis, and aging
Cellular and Molecular Life Sciences (2018)
-
Delayed APC/C activation extends the first mitosis of mouse embryos
Scientific Reports (2017)
-
The Bub1–Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation
Nature Communications (2016)