Abstract
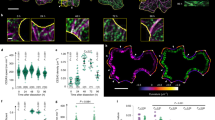
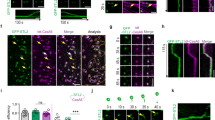
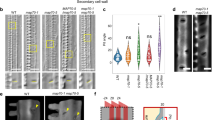
Plant cell morphogenesis relies on the organization and function of two polymer arrays separated by the plasma membrane: the cortical microtubule cytoskeleton and cellulose microfibrils in the cell wall. Studies using in vivo markers confirmed that one function of the cortical microtubule array is to drive organization of cellulose microfibrils by guiding the trajectories of active cellulose synthase (CESA) complexes in the plasma membrane, thus orienting nascent microfibrils. Here we provide evidence that cortical microtubules also position the delivery of CESA complexes to the plasma membrane and interact with small CESA-containing compartments by a mechanism that permits motility driven by microtubule depolymerization. The association of CESA compartments with cortical microtubules was greatly enhanced during osmotic stress and other treatments that limit cellulose synthesis. On recovery from osmotic stress, delivery of CESA complexes to the plasma membrane was observed in association with microtubule-tethered compartments. These results reveal multiple functions for the microtubule cortical array in organizing CESA in the cell cortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bartolini, F. & Gundersen, G. G. Generation of noncentrosomal microtubule arrays. J. Cell Sci. 119, 4155–4163 (2006).
Barton, D. A., Vantard, M. & Overall, R. L. Analysis of cortical arrays from Tradescantia virginiana at high resolution reveals discrete microtubule subpopulations and demonstrates that confocal images of arrays can be misleading. Plant Cell 20, 982–994 (2008).
Ehrhardt, D. W. Straighten up and fly right: microtubule dynamics and organization of non-centrosomal arrays in higher plants. Curr. Opin. Cell Biol. 20, 107–116 (2008).
Shaw, S. L., Kamyar, R. & Ehrhardt, D. W. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003).
Paradez, A., Wright, A. & Ehrhardt, D. W. Microtubule cortical array organization and plant cell morphogenesis. Curr. Opin. Plant Biol. 9, 571–578 (2006).
Green, P. B. Mechanism for plant cellular morphogenesis. Science 138, 1404–1405 (1962).
Heath, I. B. A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J. Theor. Biol. 48, 445–449 (1974).
Staehelin, L. A. & Giddings, T. H. Membrane mediated control of microfibrillar order, in Developmental order, its origin and regulation. (eds. S. Subtelny & P. B. Green) 133–147 (Alan R. Liss, New York; 1982).
Emons, A. M. Plasma-membrane rosettes in root hairs of Equisetum hyemale. Planta 163, 350–359 (1985).
Herth, W. Arrays of plasma-membrane 'rosettes' involved in cellulose microfibril formation of Spirogyra. Planta 159, 347–356 (1983).
Kimura, S. et al. Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell 11, 2075–2086 (1999).
Mueller, S. C. & Brown, R. M., Jr Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J. Cell Biol. 84, 315–326 (1980).
Somerville, C. Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 22, 53–78 (2006).
Desprez, T. et al. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 15572–15577 (2007).
Persson, S. et al. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc. Natl Acad. Sci. USA 104, 15566–15571 (2007).
Taylor, N. G., Howells, R. M., Huttly, A. K., Vickers, K. & Turner, S. R. Interactions among three distinct CESA proteins essential for cellulose synthesis. Proc. Natl Acad. Sci. USA 100, 1450–1455 (2003).
Paredez, A. R., Somerville, C. R. & Ehrhardt, D. W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495 (2006).
Haigler, C. H. & Brown, R. M. Transport of rosettes from the golgi apparatus to the plasma membrane in isolated mesophyll cells of Zinnia elegans during differentiation to tracheary elements in suspension culture. Protoplasma 134, 111–120 (1986).
Debolt, S. et al. Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc. Natl Acad. Sci. USA (2007).
DeBolt, S., Gutierrez, R., Ehrhardt, D. W. & Somerville, C. Nonmotile cellulose synthase subunits repeatedly accumulate within localized regions at the plasma membrane in Arabidopsis hypocotyl cells following 2, 6-dichlorobenzonitrile treatment. Plant Physiol. 145, 334–338 (2007).
Wightman, R. & Turner, S. R. The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J. 54, 794–805 (2008).
Nebenfuhr, A. et al. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 121, 1127–1142 (1999).
Uemura, T. et al. Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 29, 49–65 (2004).
Kiedaisch, B. M., Blanton, R. L. & Haigler, C. H. Characterization of a novel cellulose synthesis inhibitor. Planta 217, 922–930 (2003).
Iraki, N. M., Bressan, R. A., Hasegawa, P. M. & Carpita, N. C. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol. 91, 39–47 (1989).
Preuss, M. L., Serna, J., Falbel, T. G., Bednarek, S. Y. & Nielsen, E. The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16, 1589–1603 (2004).
Friedrichsen, D. M., Joazeiro, C. A., Li, J., Hunter, T. & Chory, J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 123, 1247–1256 (2000).
Zheng, H. et al. NPSN11 is a cell plate-associated SNARE protein that interacts with the syntaxin KNOLLE. Plant Physiol. 129, 530–539 (2002).
Nebenfuhr, A., Ritzenthaler, C. & Robinson, D. G. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 130, 1102–1108 (2002).
Bolte, S. et al. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 214, 159–173 (2004).
Bassham, D. C., Sanderfoot, A. A., Kovaleva, V., Zheng, H. & Raikhel, N. V. AtVPS45 complex formation at the trans-Golgi network. Mol. Biol. Cell 11, 2251–2265 (2000).
Hepler, P. K. & Newcomb, E. H. Microtubules and fibrils in the cytoplasm of coleus cells undergoing secondary wall deposition. J. Cell Biol. 20, 529–532 (1964).
Oda, Y., Mimura, T. & Hasezawa, S. Regulation of secondary cell wall development by cortical microtubules during tracheary element differentiation in Arabidopsis cell suspensions. Plant Physiol. 137, 1027–1036 (2005).
McFarlane, H. E., Young, R. E., Wasteneys, G. O. & Samuels, A. L. Cortical microtubules mark the mucilage secretion domain of the plasma membrane in Arabidopsis seed coat cells. Planta 227, 1363–1375 (2008).
Wu, X., Xiang, X. & Hammer, J. A. 3rd. Motor proteins at the microtubule plus-end. Trends Cell Biol. 16, 135–143 (2006).
Westermann, S. et al. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440, 565–569 (2006).
Grishchuk, E. L. et al. Different assemblies of the DAM1 complex follow shortening microtubules by distinct mechanisms. Proc. Natl Acad. Sci. USA 105, 6918–6923 (2008).
Handa, S., Bressan, R. A., Handa, A. K., Carpita, N. C. & Hasegawa, P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 73, 834–843 (1983).
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnol. 22, 1567–1572 (2004).
Earley, K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006).
Kirik, V. et al. CLASP localizes in two discrete patterns on cortical microtubules and is required for cell morphogenesis and cell division in Arabidopsis. J. Cell Sci. 120, 4416–4425 (2007).
Marc, J. et al. A GFP–MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10, 1927–1940 (1998).
Robert, S. et al. An Arabidopsis endo-1, 4-β-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell 17, 3378–3389 (2005).
Rasband, W. S. ImageJ, US National Institutes of Health, Bethesda, MD, USA .
Thevenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7, 27–41 (1998).
Sage, D., Neumann, F. R., Hediger, F., Gasser, S. M. & Unser, M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans Image Process 14, 1372–1383 (2005).
Acknowledgements
We thank S. Vernhettes, E. Nielsen and N. Geldner for providing transgenic Arabidopsis seeds; V. Kirik for help with vector construction; B. Busse for modifying the ImageJ frame alignment plugin; and S. Debolt, V. Kirik, R. Brown, T. Ketelaar and H. Höfte for helpful discussions. This work was supported by grants from the National Science Foundation (0524334) and the EU Commission (FP6-2004-NEST-C1-028974).
Author information
Authors and Affiliations
Contributions
R.G., J.L. and D.E. designed, carried out and analysed experiments; A.P. created the pCESA6::CFP::CESA6 and 35S::YFP::TUA5 transgenic lines; R.G. made all figures and movies; R.G., D.E. and A.M.E. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2271 kb)
Supplementary Information
Supplementary Movie 1 (MOV 8234 kb)
Supplementary Information
Supplementary Movie 2 (MOV 10273 kb)
Supplementary Information
Supplementary Movie 3 (MOV 12466 kb)
Supplementary Information
Supplementary Movie 4 (MOV 10485 kb)
Supplementary Information
Supplementary Movie 5 (MOV 2625 kb)
Supplementary Information
Supplementary Movie 6 (MOV 3919 kb)
Supplementary Information
Supplementary Movie 7 (MOV 4092 kb)
Supplementary Information
Supplementary Movie 8 (MOV 5018 kb)
Supplementary Information
Supplementary Movie 9 (MOV 1064 kb)
Supplementary Information
Supplementary Movie 10 (MOV 305 kb)
Supplementary Information
Supplementary Movie 11 (MOV 243 kb)
Supplementary Information
Supplementary Movie 12 (MOV 3355 kb)
Supplementary Information
Supplementary Movie 13 (MOV 12410 kb)
Supplementary Information
Supplementary Movie 14 (MOV 6700 kb)
Supplementary Information
Supplementary Movie 15 (MOV 1084 kb)
Rights and permissions
About this article
Cite this article
Gutierrez, R., Lindeboom, J., Paredez, A. et al. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11, 797–806 (2009). https://doi.org/10.1038/ncb1886
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1886
This article is cited by
-
The role of microtubules in microalgae: promotion of lipid accumulation and extraction
Biotechnology for Biofuels and Bioproducts (2023)
-
Tissue-specific directionality of cellulose synthase complex movement inferred from cellulose microfibril polarity in secondary cell walls of Arabidopsis
Scientific Reports (2023)
-
Over-expression of GhACTIN1 under the control of GhSCFP promoter improves cotton fiber and yield
Scientific Reports (2023)
-
Structure and growth of plant cell walls
Nature Reviews Molecular Cell Biology (2023)
-
Actomyosin and CSI1/POM2 cooperate to deliver cellulose synthase from Golgi to cortical microtubules in Arabidopsis
Nature Communications (2023)