Abstract
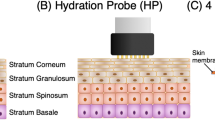
Although transdermal drug delivery is more attractive than injection, it has not been applied to macromolecules because of low skin permeability. Here we describe particular mixtures of penetration enhancers that increase skin permeability to macromolecules (∼1–10 kDa) by up to ∼100-fold without inducing skin irritation. The discovery of these mixtures was enabled by an experimental tool, in vitro skin impedance guided high-throughput (INSIGHT) screening, which is >100-fold more efficient than current tools. In vitro experiments demonstrated that the mixtures delivered macromolecular drugs, including heparin, leutinizing hormone releasing hormone (LHRH) and oligonulceotides, across the skin. In vivo experiments on hairless rats with leuprolide acetate confirmed the potency and safety of one such mixture, sodium laureth sulfate (SLA) and phenyl piperazine (PP). These studies show the feasibility of using penetration enhancers for systemic delivery of macromolecules from a transdermal patch.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zaffaroni, A. Overview and evolution of therapeutic systems. Ann. NY Acad. Sci. 618, 405–421 (1991).
Bos, J.D. & Meinardi, M.M. The 500 dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 9, 165–169 (2000).
Wertz, P.W. & Downing, D.T. in Transdermal Drug Delivery: Developmental Issues and Research Initiatives (eds. Hadgraft, J. & Guy, R.H.) 1–17 (Marcel Dekker, New York, Basel, Hong Kong, 1989).
Guy, R. et al. Iontophoresis: electrorepulsion and electroosmosis. J. Control. Release 64, 129–132 (2000).
Mitragotri, S., Blankschtein, D. & Langer, R. Ultrasound-mediated transdermal protein delivery. Science 269, 850–853 (1995).
Finnin, B.C. & Morgan, T.M. Transdermal penetration enhancers: applications, limitations and potential. J. Pharm. Sci. 88, 955–958 (1999).
Williams, A.C. & Barry, B.W. Skin absorption enhancers. Crit. Rev. Ther. Drug Carrier Syst. 9, 305–353 (1992).
French, E., Potton, C. & Walters, K. in Pharmaceutical Skin Penetration Enhancement (eds. Walters, K. & Hadgraft, J.) 113–144 (Marcel Dekker, New York, Basel, Hong Kong, 1993).
Kanikkannan, N., Kanimalla, K., Lamba, S.S. & Singh, M. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr. Med. Chem. 7, 593–608 (2000).
Lashmar, U.T., Hadgraft, J. & Thomas, N. Topical application of penetration enhancers to the skin of nude mice—a histopatholgical study. J. Pharm. Pharmacol. 41, 118–122 (1989).
Akimoto, T. & Nagase, Y. Novel transdermal drug penetration enhancer: synthesis and enhancing effect of alkyldisiloxane compounds containing glucopyranosyl group. J. Control. Release 88, 243–252 (2003).
Takanashi, Y., Higashiyama, K., Komiya, H., Takayama, K. & Nagai, T. Thiomenthol derivatives as novel percutaneous absorption enhancers. Drug Dev. Ind. Pharm. 25, 89–94 (1999).
Suhonen, M., Bouwstra, J. & Urtti, A. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Control. Release 59, 149–161 (1999).
Mitragotri, S. Effect of bilayer disruption on transdermal transport of low-molecular weight hydrophobic solutes. Pharm. Res. 18, 1022–1028 (2001).
Robinson, M.K. & Perkins, M.A. A strategy for skin irritation testing. Am. J. Contact Dermat. 13, 21–19 (2002).
Mitragotri, S. Synergistic effect of enhancers for transdermal drug delivery. Pharm. Res. 17, 1354–1359 (2000).
Funke, A.P. et al. Transdermal delivery of highly lipophilic drugs: in vitro fluxes of antiestrogens, permeation enhancers and solvents from liquid formulations. Pharm. Res. 19, 661–668 (2002).
Bronaugh, R.L. in Percutaneous Absorption: Mechanisms-Methodology-Drug Delivery (eds. Bronaugh, R.L. & Maibach, H.I.) (Marcel Dekker, New York, Basel, Hong Kong, 1989).
Karande, P. & Mitragotri, S. High-throughput screening of transdermal formulations. Pharm. Res. 19, 655–660 (2001).
Black, G. in Pharmaceutical Skin Penetration Enhancement. (eds. Walters, K. & Hadgraft, J.) 145–174 (Marcel Dekker, New York, Basel, Hong Kong, 1993).
Fentem, J.H. & Botham, P.A. ECVAM's activities in validating alternative tests for skin corrosion and irritation. Altern. Lab. Anim. 30, 61–67 (2002).
Lanigan, R.S. Final report on the safety assessment of cocoyl sarcosine, lauroyl sarcosine, myristoyl sarcosine, oleoyl sarcosine, stearoyl sarcosine, sodium cocoyl sarcosinate, sodium lauroyl sarcosinate, sodium myristoyl sarcosinate, ammonium cocoyl sarcosinate and ammonium lauroyl sarcosinate. Int. J. Toxicol. 20, 1–14 (2001).
Loffler, H. & Happle, R. Profile of irritant patch testing with detergents: sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermatitis 48, 26–32 (2003).
Berardesca, E. et al. Ranking of surfactant skin irritancy in vivo in man using the plastic occlusion stress test (POST). Contact Dermatitis 23, 1–15 (1990).
Hall-Manning, J., Holland, H., Basketter, A. & Barratt, D. Skin irritation potential of mixed surfactant systems in a human 4-hour covered patch test. Allergologie 18, 465 (1995).
Fentem, J. et al. A prevalidation study on in vitro tests for acute skin irritation: results and evaluation by the Management Team. Toxicology In Vitro 15, 57–93 (2001).
Periti, P., Mazzei, T. & Mini, E. Clinical pharmacokinetics of depot leuprorelin. Clin. Pharmacokinet. 41, 485–504 (2002).
Frydman, A. Low-molecular weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis 26, 24–38 (1996).
Peck, K., Ghanem, A. & Higuchi, W. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharm. Res. 11, 1306–1314 (1994).
Tang, H., Mitragotri, S., Blankschtein, D. & Langer, R. Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J. Pharm. Sci. 90, 543–566 (2001).
Tezel, A., Sens, A. & Mitragotri, S. A theoretical description of transdermal transport of hydrophilic solutes induced by low-frequency sonophoresis. J. Pharm. Sci. 92, 381–393 (2003).
Mitragotri, S. et al. Determination of the threshold energy dose for ultra-sound-induced transdermal drug delivery. J. Control. Release 63, 41–52 (2000).
Mitragotri, S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J. Control. Release 86, 69–92 (2003).
Acknowledgements
The authors acknowledge the technical assistance of Maricella Castaneda, Jeffery O'Neil and Drew Lassen. The authors also thank ISIS pharmaceuticals for providing oligonucleotides and Chemron for providing betaines. This research was funded in part by Cottage hospital research program, Santa Barbara, California, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The technology described in the paper is licensed to fqubed, in which S.M. is a shareholder.
Rights and permissions
About this article
Cite this article
Karande, P., Jain, A. & Mitragotri, S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol 22, 192–197 (2004). https://doi.org/10.1038/nbt928
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt928
This article is cited by
-
Biocompatible topical delivery system of high-molecular-weight hyaluronan into human stratum corneum using magnesium chloride
Scientific Reports (2023)
-
Molecular perspective of efficiency and safety problems of chemical enhancers: bottlenecks and recent advances
Drug Delivery and Translational Research (2022)
-
Praktische Aspekte der transdermalen und topischen Schmerztherapie
Schmerz Nachrichten (2021)
-
Transdermal delivery systems in cosmetics
Biomedical Dermatology (2020)
-
Correlations Between Skin Barrier Integrity and Delivery of Hydrophilic Molecules in the Presence of Penetration Enhancers
Pharmaceutical Research (2020)