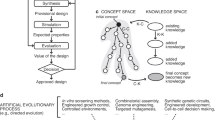
Abstract
Fermentation-based bioprocesses rely extensively on strain improvement for commercialization. Whole-cell biocatalysts are commonly limited by low tolerance of extreme process conditions such as temperature, pH, and solute concentration. Rational approaches to improving such complex phenotypes lack good models and are especially difficult to implement without genetic tools. Here we describe the use of genome shuffling to improve the acid tolerance of a poorly characterized industrial strain of Lactobacillus. We used classical strain-improvement methods to generate populations with subtle improvements in pH tolerance, and then shuffled these populations by recursive pool-wise protoplast fusion. We identified new shuffled lactobacilli that grow at substantially lower pH than does the wild-type strain on both liquid and solid media. In addition, we identified shuffled strains that produced threefold more lactic acid than the wild type at pH 4.0. Genome shuffling seems broadly useful for the rapid evolution of tolerance and other complex phenotypes in industrial microorganisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Choi, J. & Lee, S.Y. Economic considerations in the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by bacterial fermentation. Appl. Microbiol. Biotechnol. 53, 646–649 (2000).
Hashimoto, S. & Ozaki, A. Whole microbial cell processes for manufacturing amino acids, vitamins or ribonucleotides. Curr. Opin. Biotechnol. 10, 604–608 (1999).
Ho, N.W., Chen, Z., Brainard, A.P. & Sedlak, M. Successful design and development of genetically engineered Saccharomyces yeasts for effective cofermentation of glucose and xylose from cellulosic biomass to fuel ethanol. Adv. Biochem. Eng. Biotechnol. 65, 163–192 (1999).
Ikeda, M. & Katsumata, R. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose pathway. Appl. Environ. Microbiol. 65, 2497–2502 (1999).
Katsumata, R. & Ikeda, M. Hyperproduction of tryptophan in Corynebacterium glutamicum by pathway engineering. Biotechnology (NY) 11, 921–925 (1993).
Perkins, J.B. et al. Genetic engineering of Bacillus subtilis for the commercial production of riboflavin. J. Ind. Microbiol. Biotechnol. 22, 8–18 (1999).
Aristidou, A. & Penttila, M. Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 11, 187–198 (2000).
Braunegg, G., Lefebvre, G. & Genser, K.F. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65, 127–161 (1998).
Chontani, G. et al. The commercial production of chemicals using pathway engineering. Biochim. Biophys. Acta 1543, 434–455 (2000).
Donnelly, M.I., Millard, C.S., Clark, D.P., Chen, M.J. & Rathke, J.W. A novel fermentation pathway in an Escherichia coli mutant producing succinic acid, acetic acid, and ethanol. Appl. Biochem. Biotechnol. 70–72, 187–198 (1998).
Ho, N.W., Chen, Z. & Brainard, A.P. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 64, 1852–1859 (1998).
Kautola, H., Vassilev, N. & Linko, Y.Y. Continuous itaconic acid production by immobilized biocatalysts. J. Biotechnol. 13, 315–323 (1990).
Lee, S.Y. & Choi, J.I. Production of microbial polyester by fermentation of recombinant microorganisms. Adv. Biochem. Eng. Biotechnol. 71, 183–207 (2001).
Park, J.H., Kim, G.J. & Kim, H.S. Production of D-amino acid using whole cells of recombinant Escherichia coli with separately and coexpressed D-hydantoinase and N-carbamoylase. Biotechnol. Prog. 16, 564–570 (2000).
Schulze, B. & Wubbolts, M.G. Biocatalysis for industrial production of fine chemicals. Curr. Opin. Biotechnol. 10, 609–615 (1999).
Gong, C.S., Cao, N.J., Du, J. & Tsao, G.T. Ethanol production from renewable resources. Adv. Biochem. Eng. Biotechnol. 65, 207–241 (1999).
McLaren, J. & Faulkner, D. The technology roadmap for plant/crop-based renewable resources 2020 (DOE/GO-10099-706) 1–41 (US Department of Energy, Indianapolis, IN,) 1999.
Varadarajan, S. & Miller, D.J. Catalytic upgrading of fermentation-derived organic acids. Biotechnol. Prog. 15, 845–854 (1999).
Porro, D. et al. Replacement of a metabolic pathway for large-scale production of lactic acid from engineered yeasts. Appl. Environ. Microbiol. 65, 4211–4215 (1999).
Baniel, A.M. et al. Process for isolating lactic acid. US patent 6,087,532 (2000).
Rallu, F., Gruss, A., Ehrlich, S.D. & Maguin, E. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35, 517–528 (2000).
De Angelis, M., Bini, L., Pallini, V., Cocconcelli, P.S. & Gobbetti, M. The acid-stress response in Lactobacillus sanfrancisscensis CB1. Microbiology 147, 1863–1873 (2001).
Arnold, C.N., McElhanon, J., Lee, A., Leonhart, R. & Siegele, D.A. Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J. Bacteriol. 183, 2178–2186 (2001).
Audia, J.P., Webb, C.C. & Foster, J.W. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int. J. Med. Microbiol. 291, 97–106 (2001).
Arnold, F.H. & Volkov, A.A. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 3, 54–59 (1999).
Crameri, A., Raillard, S.A., Bermudez, E. & Stemmer, W.P. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 391, 288–291 (1998).
Jaeger, K.E., Eggert, T., Eipper, A. & Reetz, M.T. Directed evolution and the creation of enantioselective biocatalysts. Appl. Microbiol. Biotechnol. 55, 519–530 (2001).
Ness, J.E., Del Cardayré, S.B., Minshull, J. & Stemmer, W.P. Molecular breeding: the natural approach to protein design. Adv. Protein. Chem. 55, 261–292 (2000).
Schmidt-Dannert, C., Umeno, D. & Arnold, F.H. Molecular breeding of carotenoid biosynthetic pathways. Nat. Biotechnol. 18, 750–753 (2000).
Soong, N.W. et al. Molecular breeding of viruses. Nat. Genet. 25, 436–439 (2000).
Wackett, L.P. Directed evolution of new enzymes and pathways for environmental biocatalysis. Ann. NY Acad. Sci. 864, 142–152 (1998).
Zhang, Y., Perry, K., Powell, K., Stemmer, P.C. & del Cardayré, S.B. Evolution of Streptomyces fradiae by whole genome shuffling. Nature 415, 644–646 (2002).
Larsson, S., Quintana-Sainz, A., Reimann, A., Nilvebrant, N.O. & Jonsson, L.J. Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 84–86, 617–632 (2000).
Martinez, A., Rodriguez, M.E., York, S.W., Preston, J.F. & Ingram, L.O. Effects of Ca(OH)2 treatments (“overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol. Bioeng. 69, 526–536 (2000).
Lawford, H.G. & Rousseau, J.D. Improving fermentation performance of recombinant Zymomonas in acetic acid-containing media. Appl. Biochem. Biotechnol. 70–72, 161–172 (1998).
Zaldivar, J., Martinez, A. & Ingram, L.O. Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 68, 524–530 (2000).
Gonzalez-Vara, Y.R.A. et al. Enhanced production of L-(+)-lactic acid in chemostat by Lactobacillus casei DSM 20011 using ion-exchange resins and cross-flow filtration in a fully automated pilot plant controlled via NIR. Biotechnol. Bioeng. 67, 147–156 (2000).
Benninga, H.A. A History of Lactic Acid Making (Kluyver Academic Publishers, Dordrecht, The Netherlands, 1990).
Hongo, M., Nomura, Y. & Iwahara, M. Novel methods of lactic production by electrodialysis fermentation. Appl. Environ. Microbiol. 32, 227–234 (1986).
O'Sullivan, E. & Condon, S. Intracellular pH is a major factor in the induction of tolerance to acid and other stresses in Lactococcus lactis. Appl. Environ. Microbiol. 63, 4210–4215 (1997).
Del Cardayré, S.B. et al. Molecular breeding of transposable elements. PCT application WO 02/04629 A (2002).
Miller, J.H. in Experiments in Molecular Genetics. (ed. Miller, J.H.) 125–129 (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1972).
Cocconcelli, P.S., Morelli, L., Vescovo, M. & Bottazzi, V. Intergeneric protoplast fusion in lactic acid bacteria. FEMS Microbiol. Lett. 35, 211–214 (1986).
Morelli, L. et al. Lactobacillus protoplast transformation. Plasmid 17, 73–75 (1987).
Acknowledgements
We thank Keith Powell and Tony Cox for their useful suggestions and discussions and Ken Zahn for proofreading the manuscript. Special thanks go to Amy Giver, Henry Garcia, and Rob Pak for technical support. Funding for this project was provided in part by the US National Institute of Standards and Technology Advanced Technology Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Each of the authors is or was an employee of Maxygen, Codexis, or Cargill Dow, LLC.
Rights and permissions
About this article
Cite this article
Patnaik, R., Louie, S., Gavrilovic, V. et al. Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20, 707–712 (2002). https://doi.org/10.1038/nbt0702-707
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0702-707
This article is cited by
-
Enhanced ε‑poly‑l‑lysine production in Streptomyces species by combining interspecific hybridization with multiple antibiotic resistance
Bioprocess and Biosystems Engineering (2024)
-
Development of lead (Pb) tolerant strain by protoplast technology and their remediation
World Journal of Microbiology and Biotechnology (2023)
-
Identification of a major facilitator superfamily protein that is beneficial to L-lactic acid production by Bacillus coagulans at low pH
BMC Microbiology (2022)
-
Enterococcus faecium PNC01 isolated from the intestinal mucosa of chicken as an alternative for antibiotics to reduce feed conversion rate in broiler chickens
Microbial Cell Factories (2021)
-
Combining Genome Shuffling with Streptomycin Resistance to Improve Poly-γ-L-diaminobutanoic Acid Production in Bacillus pumilus
Biotechnology and Bioprocess Engineering (2021)