Abstract
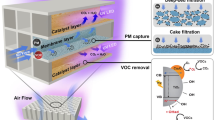
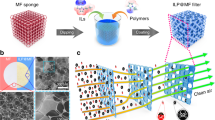
The adverse impact of particulate air pollution on human health1,2 has prompted the development of purification systems that filter particulates out of air3,4,5. To maintain performance, the filter units must inevitably be replaced at some point, which requires maintenance, involves costs and generates solid waste6,7. Here we show that an ion-doped conjugated polymer-coated matrix infiltrated with a selected functional liquid enables efficient, continuous and maintenance-free air purification. As the air to be purified moves through the system in the form of bubbles, the functional fluid provides interfaces for filtration and for removal of particulate matter and pollutant molecules from air. Theoretical modelling and experimental results demonstrate that the system exhibits high efficiency and robustness: its one-time air purification efficiency can reach 99.6%, and its dust-holding capacity can reach 950 g m−2. The system is durable and resistant to fouling and corrosion, and the liquid acting as filter can be reused and adjusted to also enable removal of bacteria or odours. We anticipate that our purification approach will be useful for the development of specialist air purifiers that might prove useful in a settings such as hospitals, factories and mines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author.
References
Nel, A. Air pollution-related illness: effects of particles. Science 308, 804–806 (2005).
Dedoussi, I. C., Eastham, S. D., Monier, E. & Barrett, S. R. H. Premature mortality related to United States cross-state air pollution. Nature 578, 261–265 (2020).
Stanford, M. G. et al. Self-sterilizing laser-induced graphene bacterial air filter. ACS Nano 13, 11912–11920 (2019).
Zhang, G. H. et al. High-performance particulate matter including nanoscale particle removal by a self-powered air filter. Nat. Commun. 11, 1653 (2020).
Wang, Q. et al. Polarity-dominated stable N97 respirators for airborne virus capture based on nanofibrous membranes. Angew. Chem. Int. Ed. 60, 23756–23762 (2021).
Zhang, Y. et al. Preparation of nanofibrous metal-organic framework filters for efficient air pollution control. J. Am. Chem. Soc. 138, 5785–5788 (2016).
Liu, G. L. et al. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 32, 375–396 (2017).
Liu, C. et al. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 6, 6205 (2015).
Li, M. et al. Novel hollow fiber air filters for the removal of ultrafine particles in PM2.5 with repetitive usage capability. Environ. Sci. Technol. 51, 10041–10049 (2017).
Zhong, Z. et al. Unusual air filters with ultrahigh efficiency and antibacterial functionality enabled by ZnO nanorods. ACS Appl. Mater. Interfaces 7, 21538–21544 (2015).
Omota, F., Dimian, A. C. & Bliek, A. Adhesion of solid particles to gas bubbles. Part 1: modelling. Chem. Eng. Sci. 61, 823–834 (2006).
Dedovets, D. et al. Multiphase microreactors based on liquid-liquid and gas-liquid dispersions stabilized by colloidal catalytic particles. Angew. Chem. Int. Ed. 60, 2–25 (2022).
Zhang, M. & Guiraud, P. Surface-modified microbubbles (colloidal gas aphrons) for nanoparticle removal in a continuous bubble generation-flotation separation system. Water Res. 126, 399–410 (2017).
Wesley, D. J., Smith, R. M., Zimmerman, W. B. & Howse, J. R. Influence of surface wettability on microbubble formation. Langmuir 32, 1269–1278 (2016).
Brown, K. L. & Wray, S. in Hygiene in Food Processing (eds Lelieveld, H. L. M., Holah, J. T. & Napper, D.) 174–202 (Woodhead Publishing, 2014).
Agarwal, A., Ng, W. J. & Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84, 1175–1180 (2011).
Hou, X., Hu, Y., Grinthal, A., Khan, M. & Aizenberg, J. Liquid-based gating mechanism with tunable multiphase selectivity and antifouling behaviour. Nature 519, 70–73 (2015).
Alvarenga, J. et al. Research Update: Liquid gated membrane filtration performance with inorganic particle suspensions. APL Mater. 6, 100703 (2018).
Mao, X. W. et al. Energetically efficient electrochemically tunable affinity separation using multicomponent polymeric nanostructures for water treatment. Energ. Environ. Sci. 11, 2954–2963 (2018).
Pich, J. & Schutz, W. On the theory of particle deposition in rising gas-bubbles: the absorption minimum. J. Aerosol Sci. 22, 267–272 (1991).
Wei, Y., Zhang, Q. & Thompson, J. E. The wetting behavior of fresh and aged soot studied through contact angle measurements. Atmos. Clim. Sci. 7, 11–22 (2017).
Binks, B. P. & Lumsdon, S. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 16, 8622–8631 (2000).
Kumar, B., Patil, A. J. & Mann, S. Enzyme-powered motility in buoyant organoclay/DNA protocells. Nat. Chem. 10, 1154–1163 (2018).
Li, P. et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 10, 2177 (2019).
Tian, E. et al. Ultralow resistance two-stage electrostatically assisted air filtration by polydopamine coated PET coarse filter. Small 17, e2102051 (2021).
Xiao, R., Mo, J., Zhang, Y. & Gao, D. An in-situ thermally regenerated air purifier for indoor formaldehyde removal. Indoor Air 28, 266–275 (2018).
Lu, W. et al. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 51, 7480–7484 (2012).
Van Harmelen, A., Kok, H. & Visschedijk, A. Potentials and Costs to Reduce PM10 and PM2.5 Emissions from Industrial Sources in the Netherlands (TNO Environment, Energy and Process Innovation, 2002).
Chen, L., Li, P., Liu, G., Cheng, W. & Liu, Z. Development of cement dust suppression technology during shotcrete in mine of China – a review. J. Loss Prev. Process Ind. 55, 232–242 (2018).
Zhu, M. M. et al. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 302, 1600353 (2017).
Liu, M., Nie, F. Q., Wei, Z., Song, Y. & Jiang, L. In situ electrochemical switching of wetting state of oil droplet on conducting polymer films. Langmuir 26, 3993–3997 (2010).
Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. London 95, 65–87 (1805).
Owens, D. K. & Wendt, R. C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 13, 1741–1747 (1969).
Kape, A., Ruick, B. & Drusch, S. Characterisation of the work of adhesion of food grade coating materials on a maltodextrin model surface. Chem. Eng. Res. Des. 110, 152–159 (2016).
Harris, A. F. & Beevers, A. The effects of grit-blasting on surface properties for adhesion. Int. J. Adhes. Adhes. 19, 445–452 (1999).
Kwon, G. et al. On-demand separation of oil-water mixtures. Adv. Mater. 24, 3666–3671 (2012).
Liu, C. J., Liang, B., Tang, S. W. & Min, E. Z. Effects of orifice orientation and gas-liquid flow pattern on initial bubble size. Chin. J. Chem. Eng. 21, 1206–1215 (2013).
Longuet-Higgins, M. S., Kerman, B. R. & Lunde, K. The release of air bubbles from an underwater nozzle. J. Fluid Mech. 230, 365–390 (1991).
Higuera, F. J. Injection and coalescence of bubbles in a very viscous liquid. J. Fluid Mech. 530, 369–378 (2005).
Ghiaasiaan, S. M. & Yao, G. F. A theoretical model for deposition of aerosols in rising spherical bubbles due to diffusion, convection, and inertia. Aerosol Sci. Technol. 26, 141–153 (1997).
Charvet, A., Bardin-Monnier, N. & Thomas, D. Can bubble columns be an alternative to fibrous filters for nanoparticles collection? J. Hazard. Mater. 195, 432–439 (2011).
Ghiaasiaan, S. M. & Yao, G. F. Diffusive and convective deposition of aerosols in rising spherical bubbles with internal circulation. Int. J. Multiphase Flow 21, 907–918 (1995).
Licht, W. The movement of aerosol particles. J. Soc. Cosmet. Chem. 23, 657–678 (1972).
Larmour, I. A., Bell, S. E. & Saunders, G. C. Remarkably simple fabrication of superhydrophobic surfaces using electroless galvanic deposition. Angew. Chem. Int. Ed. 46, 1710–1712 (2007).
Liu, M., Wang, S. & Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2, 17036 (2017).
Binks, B. P. Particles as surfactants – similarities and differences. Curr. Opin. Colloid Interface Sci. 7, 21–41 (2002).
Wallis, G. B. The terminal speed of single drops or bubbles in an infinite medium. Int. J. Multiphase Flow 1, 491–511 (1974).
Tan, K. L., Tan, B. T. G., Kang, E. T. & Neoh, K. G. The chemical nature of the nitrogens in polypyrrole and polyaniline: a comparative-study by x-ray photoelectron-spectroscopy. J. Chem. Phys. 94, 5382–5388 (1991).
Tan, X., Hu, C., Zhu, Z., Liu, H. & Qu, J. Electrically pore-size-tunable polypyrrole membrane for antifouling and selective separation. Adv. Funct. Mater. 29, 1903081 (2019).
Zhang, H. et al. Design of three-dimensional gradient nonwoven composites with robust dust holding capacity for air filtration. J. Appl. Polym. Sci. 136, 47827 (2019).
Liu, J. et al. Low resistance bicomponent spunbond materials for fresh air filtration with ultra-high dust holding capacity. RSC Adv. 7, 43879–43887 (2017).
Liu, J. et al. Polyethylene/polypropylene bicomponent spunbond air filtration materials containing magnesium stearate for efficient fine particle capture. ACS Appl. Mater. Interfaces 11, 40592–40601 (2019).
Liu, B., Zhang, S., Wang, X., Yu, J. & Ding, B. Efficient and reusable polyamide-56 nanofiber/nets membrane with bimodal structures for air filtration. J. Colloid Interface Sci. 457, 203–211 (2015).
Li, Y. Y. et al. Semi-interpenetrating polymer network biomimetic structure enables superelastic and thermostable nanofibrous aerogels for cascade filtration of PM2.5. Adv. Funct. Mater. 30, 1910426 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 52025132, 21975209, 21621091, 22021001 and 22121001), the National Key R&D Programme of China (grant no. 2018YFA0209500), the 111 Project (grant nos. B17027 and B16029) and the Science and Technology Projects of Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (grant no. RD2022070601). We thank L. Huang, L. Min, B. Chen, H. Liu, D. Wu and K. Zhan for their support and discussion of the synthesis and measurements of the materials.
Author information
Authors and Affiliations
Contributions
X.H. conceived the project and designed the research. Y.Z. and S.W. synthesized the materials. Y.Z., Y. Han, Z.S., Y. Hou, C.W. and M.W. prepared and characterized the samples, and analysed the data. Y.Z., L.Q. and Y. Han performed the pressure, microbubble size and air purification experiments and analysed the data. Y.Z., X.J., D.Z., Z.S. and L.Q. built the mathematical model. Y.Z, X.C. and X.H. wrote the manuscript. All the authors contributed to the data analysis, discussion and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Pascal Guiraud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The electrochemical polymerization of doped PPy.
a. The photo of the electrochemical polymerization setup. b. Current density dependent electrochemical polymerization time. Inset: Charge density dependent electrochemical polymerization time. c. The optical images of doped PPy coated SSM at different polymerization times. d. SEM images of doped PPy coated SSM at different polymerization times.
Extended Data Fig. 2 The surface composition analysis of DPM in the redox process.
a. Cyclic voltammetry (CV) curve of DPM in 0.1 M LiClO4 at a scan rate of 50 mV s−1. The curve shows the reversibility of the redox process with an oxidization peak at 0.12 V and a reduction peak at − 0.39 V. b. The X-ray photoelectron spectroscopy (XPS) survey scans of DPM in different states, which prove the matrix surface composition changes in the redox process. c, d. High-resolution Li 1s XPS spectra of DPM in the reduction state (c) and the oxidation state (d), which show the Li+ ions doping and dedoping during the redox processes. e, f. High-resolution N 1s XPS spectra of DPM in the reduction state (e) and the oxidation state (f). Four types of nitrogen environments are present in DPM. The higher peaks at 401.4 eV and 402.9 eV can be assigned to the positively charged –HN+– and bipolarons moieties48. The positively charged N moieties are higher in the oxidation state, which indicates that the redox reaction have occurred on the DPM.
Extended Data Fig. 3 The wettability and morphology of the DPF in the redox process.
a. The self-designed setup for in situ monitoring the contact angle of a dichloromethane droplet on the DPF during adjusting the electrochemical potentials underwater. A three-electrode system was used to control the electrochemical potential. DPF was served as the working electrode. Ag/AgCl electrode and a platinum plate were used as the reference electrode and the counter electrode, respectively. 0.1 M LiClO4 aqueous solution was used as the electrolyte. b. Reversible wettability switching of the functional liquid on DPF during the continuous cycle redox process. To obtain a wide range of wettability values with a small potential difference, we selected +0.40 V and −0.60 V as the optimal working oxidation and reduction potentials, respectively, based on the contact angle analysis at different potentials in Fig. 2b. Error bars represent the standard deviation from five independent measurements. c. Underwater wettability switching of oil on DPF during the cycle redox process. Error bars represent the standard deviation from five independent measurements. d, e. Analysis of the adhesion work between the functional liquid and the matrix in reduction state (d) and oxidation state (e): the polar part of the surface tension/energy and the corresponding surface tension/energy can be identified and the combination of them provides the optimum adhesion between DPF and functional liquid. The blue and red straight line represents the optimum adhesive force, and a range of acceptable performance can be proposed. From the results, one can conclude that the functional liquid falls in the optimum zone in the reduction state, which indicates that the affinity between the functional liquid and the matrix adheres well. f, g. 3D atomic force microscope (AFM) images show height variations on the surface of the matrix in reduction state (f) and oxidation state (g). AFM results suggest that the surface of the matrix changes from smooth to rough during the reduction process due to the influx of lithium ions49, which could also increase the hydrophilicity of the surface.
Extended Data Fig. 4 The SEM images of the filtrated DPM after backwashing.
a. DPM in oxidation state without liquid-lining; b. DPM from ELBS in oxidation state with liquid-lining; c. DPM in reduction state without liquid-lining; d. DPM from ELBS in reduction state with liquid-lining; Scale bar, 100 μm. After the backwashing, we can find that few particles exist on the liquid-lined matrix while many particles exist on the matrix without liquid, which demonstrate that the liquid-lining improves the anti-fouling property of the matrix. In addition, we can use the functional liquid to regulate the wettability of the matrix, which will influence the affinity among the contaminant, functional liquid, and matrix and further prevent particle accumulation. The surface of the matrix with liquid-lining in reduction state was cleaner than that in oxidation state, because of the stronger affinity between the lined liquid and the reduction state matrix. Therefore, we can apply different potentials to change the wettability of the matrix to reduce the affinity to further prevent particles accumulation. In addition, once the regulation is completed, the redox state of the liquid-based matrix can be maintained all the time without continuous power input.
Extended Data Fig. 5 Transport properties and microbubbles generation of ELBS.
a. Optical image of the pressure measurement setup. b. Schematic of the pressure-induced bulge of the liquid-air interface. Here, L is the pore size of DPM, R is the radius of the cylindrical fiber in DPM, Rsag is the critical radius of curvature of the liquid-air interface, θ is Young’s contact angle, ψ is the texture angle, δθ is the convex angle. c. Theoretical and experimental value of critical pressure (Pc) of air passing through ELBS with different pore size matrices during the redox process. The error bars represent the standard deviation from five independent measurements. d. The theoretical maximum and minimum and experimental diameters of microbubbles generated from ELBS with different matrix pore sizes in the redox process. Error bars represent the standard deviation from five independent measurements. e. The sizes of the microbubbles generated from ELBS with the average pore size of ~9.7 μm in the repeating redox process. Error bars represent the standard deviation from ten independent bubble measurements.
Extended Data Fig. 6 The air purification performance of the ELBS.
a. Adhesion of the hydrophobic particles to the gas–liquid interface. b. Representation of the forces acting on the hydrophobic particle at the bubble interface. Here, Rp is the radius of the particle, Rb is the radius of the bubble, FP is the force resulting from the capillary pressure inside the gas bubble, FB is the buoyancy force, FG is the gravitational force, Fγ is the surface tension force of the liquid. c. Optical image of the in situ study customized particulate matter (PM) purification system. d. The schematic of the purification system and the capture process of the droplets generated from the bursting bubbles. e. The particle size distribution in the liquid in the inlet side and outlet side. There are two purification steps: the direct interception and removal of larger PM in the polluted air by the liquid-lined porous matrix (inlet side), and the capture of smaller PM by the functional liquid through the microbubble interface (outlet side).
Extended Data Fig. 7
Optical photographs of DPM, SSP (stainless-steel plate), and SSM after immersed in 1.0 M NaOH solutions, 0.5 M H2SO4 solutions, 1.0 M NaCl solutions, at different temperatures for 24 h, respectively. Scale bar, 1 cm.
Supplementary information
Supplementary Video 1
In situ observation of PPy growing on SSM.
Supplementary Video 2
In situ observation of microbubble generation from ELBS. DPM is the working electrode, Au/SSM is the counter electrode, Ag/AgCl is the reference electrode and LiClO4 aqueous solution is the functional liquid.
Supplementary Video 3
Adhesion of different wettability particles to different gas–liquid interfaces.
Supplementary Video 4
Demonstration of using ELBS to absorb PM in a larger environment.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Han, Y., Ji, X. et al. Continuous air purification by aqueous interface filtration and absorption. Nature 610, 74–80 (2022). https://doi.org/10.1038/s41586-022-05124-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05124-y
This article is cited by
-
Ultrathin, ultralight dual-scale fibrous networks with high-infrared transmittance for high-performance, comfortable and sustainable PM0.3 filter
Nature Communications (2024)
-
Bifunctional Activated Carbon Ultrathin Fibers: Combining the Removal of VOCs and PM in One Material
Advanced Fiber Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.