Abstract
The origin of tetrapods from their fish antecedents, approximately 400 million years ago, was coupled with the origin of terrestrial locomotion and the evolution of supporting limbs. Polypterus is a member of the basal-most group of ray-finned fish (actinopterygians) and has many plesiomorphic morphologies that are comparable to elpistostegid fishes, which are stem tetrapods. Polypterus therefore serves as an extant analogue of stem tetrapods, allowing us to examine how developmental plasticity affects the ‘terrestrialization’ of fish. We measured the developmental plasticity of anatomical and biomechanical responses in Polypterus reared on land. Here we show the remarkable correspondence between the environmentally induced phenotypes of terrestrialized Polypterus and the ancient anatomical changes in stem tetrapods, and we provide insight into stem tetrapod behavioural evolution. Our results raise the possibility that environmentally induced developmental plasticity facilitated the origin of the terrestrial traits that led to tetrapods.
This is a preview of subscription content, access via your institution
Access options




Similar content being viewed by others
Change history
03 September 2014
The Fig. 4 legend has been updated.
References
Niedźwiedzki, G., Szrek, P., Narkiewicz, K., Narkiewicz, M. & Ahlberg, P. E. Tetrapod trackways from the early Middle Devonian period of Poland. Nature 463, 43–48 (2010)
Clack, J. A. The fin to limb transition: new data, interpretations, and hypotheses from paleontology and developmental biology. Annu. Rev. Earth Planet. Sci. 37, 163–179 (2009)
Callier, V., Clack, J. A. & Ahlberg, P. E. Contrasting developmental trajectories in the earliest known tetrapod forelimbs. Science 324, 364–367 (2009)
Shubin, N. H., Daeschler, E. B. & Jenkins, F. A. J. The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440, 764–771 (2006)
Pierce, S. E., Clack, J. A. & Hutchinson, J. R. Three-dimensional limb joint mobility in the early tetrapod Ichthyostega. Nature 486, 523–527 (2012)
Ahlberg, P. E. & Clack, J. A. Palaeontology: a firm step from water to land. Nature 440, 747–749 (2006)
Clack, J. A. Gaining Ground 2nd edn (Indiana Univ. Press, 2012)
Coates, M. I. Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution. Trans. R. Soc. Edinb. Earth Sci. 87, 363–421 (1996)
Coates, M. I., Ruta, M. & Friedman, M. Ever since Owen: changing perspectives on the early evolution of tetrapods. Annu. Rev. Ecol. Evol. Syst. 39, 571–592 (2008)
Blob, R. W. & Biewener, A. A. Mechanics of limb bone loading during terrestrial locomotion in the green iguana (Iguana iguana) and American alligator (Alligator mississippiensis). J. Exp. Biol. 204, 1099–1122 (2001)
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford Univ. Press, 2003)
Moczek, A. P. et al. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B 278, 2705–2713 (2011)
Ghalambor, C. K., McKay, J. K., Carroll, S. P. & Reznick, D. N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (2007)
Price, T. D., Qvarnstrom, A. & Irwin, D. E. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440 (2003)
Yeh, P. J. & Price, T. D. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 164, 531–542 (2004)
Crispo, E. The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61, 2469–2479 (2007)
Waddington, C. H. Genetic assimilation of an acquired character. Evolution 7, 118–126 (1953)
Gomez-Mestre, I. & Buchholz, D. R. Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proc. Natl Acad. Sci. USA 103, 19021–19026 (2006)
Wund, M. A., Baker, J. A., Clancy, B., Golub, J. L. & Foster, S. A. A test of the “flexible stem” model of evolution: ancestral plasticity, genetic accommodation, and morphological divergence in the threespine stickleback radiation. Am. Nat. 172, 449–462 (2008)
Rajakumar, R. et al. Ancestral developmental potential facilitates parallel evolution in ants. Science 335, 79–82 (2012)
Inoue, J. G., Miya, M., Tsukamoto, K. & Nishida, M. Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the “ancient fish”. Mol. Phylogenet. Evol. 26, 110–120 (2003)
Tucker, V. A. The energetic cost of moving about: walking and running are extremely inefficient forms of locomotion. Much greater efficiency is achieved by birds, fish—and bicyclists. Am. Sci. 63, 413–419 (1975)
Kawano, S. M. & Blob, R. W. Propulsive forces of mudskipper fins and salamander limbs during terrestrial locomotion: Implications for the invasion of land. Integr. Comp. Biol. 53, 283–294 (2013)
Gosline, W. A. The structure and function of the dermal pectoral girdle in bony fishes with particular reference to ostariophysines. J. Zool. 183, 329–338 (1977)
Winterbottom, R. A descriptive synonymy of the striated muscles of the teleostei. Proc. Acad. Nat. Sci. Philadelphia 125, 225–317 (1973)
Biewener, A. A. Scaling body support in mammals: limb posture and muscle mechanics. Science 245, 45–48 (1989)
Frost, H. M. On our age-related bone loss: insights from a new paradigm. J. Bone Miner. Res. 12, 1539–1546 (1997)
Andrews, S. M. & Westoll, T. S. The postcranial skeleton of Eusthenopteron foorii Whiteaves. Trans. R. Soc. Edinb. 68, 207–329 (1970)
Badyaev, A. V. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R. Soc. B 272, 877–886 (2005)
Suzuki, Y. & Nijhout, H. F. Evolution of a polyphenism by genetic accommodation. Science 311, 650–652 (2006)
Lerouzic, A. & Carlborg, O. Evolutionary potential of hidden genetic variation. Trends Ecol. Evol. 23, 33–37 (2008)
Bartsch, P., Gemballa, S. & Piotrowski, T. The embryonic and larval development of Polypterus senegalus Cuvier, 1829: its staging with reference to external and skeletal features, behaviour and locomotory habits. Acta Zool. 78, 309–328 (1997)
Reinschmidt, C. & van den Bogert, T. KineMat: a MATLAB toolbox for three-dimensional kinematic analyses. http://isbweb.org/software/movanal/kinemat/ (Human Performance Laboratory, Univ. Calgary, 1997)
Hedrick, T. L. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 (2008)
Zar, J. H. Biostatistical Analysis. 4th edn (Prentice Hall, 1999)
Batschelet, E. Statistical Methods for the Analysis of Problems in Animal Orientation and Certain Biological Rhythms (American Institute of Biological Sciences, 1965)
Batschelet, E. Circular Statistics in Biology (Academic, 1981)
Brechbühler, C. H., Gerig, G. & Kübler, O. Parametrization of closed surfaces for 3-D shape description. Comput. Vis. Image Underst. 61, 154–170 (1995)
Shen, L., Farid, H. & McPeek, M. A. Modeling three-dimensional morphological structures using spherical harmonics. Evolution 63, 1003–1016 (2009)
Styner, M. et al. Framework for the statistical shape analysis of brain structures using SPHARM-PDM. Insight J. http://www.insight-journal.org/browse/publication/101 (11 July 2006)
Paniagua, B., Styner, M., Macenko, M., Pantazis, D. & Niethammer, M. Local shape analysis using MANCOVA. Insight J. http://www.insight-journal.org/browse/publication/694 (10 September 2009)
Acknowledgements
We thank F. A. Jenkins Jr and C. R. Marshall for encouragement and discussions with E.M.S. during the initial phases of this project. We also thank E. Abouheif for discussions and editing of the manuscript. We thank J. Dawson for the loan of camera equipment, S. Bertram for access to laboratory space while running the experiment and B. Bongfeldt for animal care and preliminary data analysis. We are grateful for the Tomlinson Post-doctoral Fellowship (E.M.S.), for grants from the National Sciences and Engineering Research Council of Canada (PDF to E.M.S., CGS-M to T.Y.D. and Discovery Grant #261796-2011 to H.C.E.L.), for the Robert G. Goelet Research Award (E.M.S.) and to Canada Research Chairs (H.C.E.L.).
Author information
Authors and Affiliations
Contributions
E.M.S. conceived, designed and conducted the experiments and biomechanical analyses. T.Y.D. scanned, segmented and analysed the micro-computed tomography images. H.C.E.L. provided palaeontological and evolutionary expertise that shaped the project. E.M.S., T.Y.D. and H.C.E.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Timing of kinematic variables for the left fin during swimming in control and treatment group fish.
The polar plot represents a complete stroke cycle, starting at 0° with the fin fully adducted. At mid-stroke (180°), the fin is fully abducted, and then it adducts again (360°/0°). The data are plotted for the treatment group (dark grey, n = 12) and the control group (light grey, n = 6). Only data with significant directionality are plotted (Rayleigh’s test, P < 0.05). If the groups did not differ significantly in the timing of a given variable, they were binned and plotted together (black). The symbols represent the mean timing ( ± angular variance) of different kinematic variables.
Extended Data Figure 2 Variance in bone shape in control and treatment group fish.
Bone-shape variances between the control group (light grey, n = 7) and the treatment group (dark grey, n = 15). Dots indicate observed variance; error bars indicate bootstrapped 95% confidence intervals.
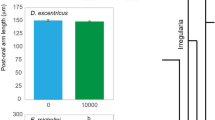
Extended Data Figure 3 Body length and mass in control and treatment group fish.
Bootstrapped differences in mean body length and mass between water-raised and land-raised fish at the start (a) and end (b) of the experiment. At the start of the experiment, land-raised fish were smaller than water-raised fish (Extended Data Table 6). Similar size relationships existed between the control (light grey) and treatment (dark grey) groups at the end of the experiment, but the differences were much greater (Extended Data Table 6). Length and weight data at the start (control, n = 38; treatment, n = 111) and the end (control, n = 30; treatment, n = 69) of the experiment were bootstrapped 10,000 times, and means were generated from each bootstrap. These values were used to test how the mean difference between the groups changed from the start to the end of the experiment. In all cases, the differences between the control and treatment groups increased over time.
Supplementary information
Polypterus senegalus walking on a smooth surface
This video is slowed down by ten times. (MOV 15155 kb)
Rights and permissions
About this article
Cite this article
Standen, E., Du, T. & Larsson, H. Developmental plasticity and the origin of tetrapods. Nature 513, 54–58 (2014). https://doi.org/10.1038/nature13708
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13708
This article is cited by
-
Early onset of urea synthesis and ammonia detoxification pathways in three terrestrially developing frogs
Journal of Comparative Physiology B (2023)
-
Darwin’s agential materials: evolutionary implications of multiscale competency in developmental biology
Cellular and Molecular Life Sciences (2023)
-
Long lasting effects of early temperature exposure on the swimming performance and skeleton development of metamorphosing Gilthead seabream (Sparus aurata L.) larvae
Scientific Reports (2021)
-
Adaptations of Interferon Regulatory Factor 3 with Transition from Terrestrial to Aquatic Life
Scientific Reports (2020)
-
Elpistostege and the origin of the vertebrate hand
Nature (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.